The global regulatory information management market size was valued at US$ 1,680.0 million in 2022 and is anticipated to witness a compound annual growth rate (CAGR) of 11.4% from 2023 to 2030.
Regulatory information management software consists of software solutions suiting the respective industry and nature of business, especially for pharmaceutical, biotechnology, and clinical research industries. The software allows manufacturers and respective personnel in the aforementioned industries to ensure strict observance of complex regulations set by regulatory authorities in the respective regions.
Global Regulatory Information Management Market: Regional Insights
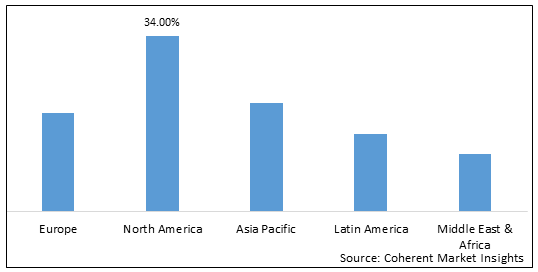
The executive's framework market can be divided geographically into North America, Europe, Asia Pacific, the Middle East and Africa, and South America. Due to the presence of drug stalwarts and deeply rooted and mechanically advanced organizations in the United States and Canada, North America dominated the global administrative data the executive's framework market in 2022.
In view of the U.S. besides, Canada's profoundly grounded and precisely advanced drug endeavors as well as the developing clinical contraption region, North America is conceivable the one to see a noteworthy presence in the overall managerial information the leader's system market in 2022. Additionally, a number of vendors of regulatory information management systems provide integrated regulatory solutions like regulatory information management and Regulatory submission, among others. North America held a share of over 34% in 2022.
In the U.S., it is moving for associations to really follow managerial changes, collect, organize, and pass thing information on to authoritative specialists in various countries due to the novel overall regulatory scene, manual business frameworks, complex endeavor conditions, and resource limitations.
Figure 1. Global Regulatory Information Management Market Share (%), By Region, 2022
To learn more about this report, request a free sample copy
Global Regulatory Information Management Market Drivers:
Regulatory compliance requirements
Businesses across various sectors, such as pharmaceuticals, healthcare, finance, and manufacturing, must comply with a multitude of regulations imposed by government authorities. These regulations often involve extensive documentation, reporting, and data management. Regulatory information management systems help companies streamline these processes, ensuring compliance and minimizing the risk of non-compliance penalties. Dovel Technologies, ltd., develops solutions for monitoring and analyzing public health data to better inform solutions for effective response and proactive prevention efforts.
Evolving regulatory landscape
The regulatory environments are always changing, with new regulations being added and old ones being improved. It is difficult for businesses to effectively manage their compliance efforts and keep up with the ever-changing nature of regulations. Edge arrangements offer functionalities like following administrative refreshed, robotizing consistence processes, and giving constant admittance to refreshed administrative data.
Global Regulatory Information Management Market Opportunities:
As the regulatory framework evolves and becomes more stringent in emerging markets, there is a growing need for effective regulatory information management solutions. Countries that are experiencing rapid industrialization and increased regulatory oversight offer significant opportunities for regulatory information management vendors to provide compliance management solutions tailored to local requirements.
The integration of regulatory information management systems with emerging technologies such as artificial intelligence (AI), Machine Learning (ML), robotics process automation (RPA), and natural language processing (NLP) presents opportunities for enhanced automation, data analysis, and decision-making capabilities. Intelligent regulatory information management solutions can streamline compliance processes, improve data accuracy, and enable proactive compliance management.
Regulatory Information Management Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2022 | Market Size in 2022: | US$ 1,680.0 Mn |
| Historical Data for: | 2017 to 2022 | Forecast Period: | 2023 to 2030 |
| Forecast Period 2023 to 2030 CAGR: | 11.4% | 2030 Value Projection: | US$ 3,973.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Major players operating in the global regulatory information management market include Acuta, Llc, Parexel, Computer Sciences Corp (CSC), Aris Global LLC, Virtify, Ennov, Amplexor, Samarind Ltd, Dovel Technologies, Inc., and Informa |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Global Regulatory Information Management Market- Impact of Coronavirus (Covid-19) Pandemic
Every nation's economy was suffering as a result of COVID-19. Developed nations are severely impacted by this pandemic. Organizations in many countries endured because of either a fractional or complete lockdown. During the pandemic, borders around the world were closed, which hurt the supply chain industry a lot. COVID-19, on the other hand, helped the market for regulatory information management systems.
WHO reports that more than 300 clinical trials have begun in search of a COVID-19 treatment. The majority of trials were conducted by China and South Korea. Nearly 500 clinical preliminary trials for the Coronavirus have been registered as of April 21, 2020. Additionally, in April 2020, Oracle constructed and donated a COVID-19 Therapeutic Learning System to the US government. Patients and doctors alike can use this system to monitor the effectiveness of promising COVID-19 drug therapies. As a result, the board frameworks require more administrative data as the number of clinical preliminary exams rises during the pandemic.
Global Regulatory Information Management Market Trends:
Increasing focus on data governance and data integrity
Regulatory authorities are placing greater emphasis on data governance and data integrity in industries such as pharmaceuticals, healthcare, and financial services. Regulatory information management solutions that offer robust data management capabilities, data validation, audit trails, and data integrity checks are in high demand to ensure the accuracy, reliability, and traceability of regulatory information. In July 2021, Pharmaceutical Inspection Cooperation Scheme (PIC/S) entitled Good Practices for Data Management and Integrity in Regulated GMP/GDP Environments. This article will give an overview of the whole guidance document and review specific requirements for computerized systems.
Adoption of artificial intelligence and automation
The adoption of artificial intelligence and automation technologies is gaining momentum in regulatory information management market. AI-powered regulatory information management solutions can automate manual tasks, improve data processing efficiency, enhance compliance analytics, and enable predictive capabilities. Automation reduces human errors, saves time, and enables organizations to focus on strategic regulatory initiatives.
Global Regulatory Information Management Market Restraints:
Data privacy and security concerns
Handling sensitive regulatory data, such as patient data, intellectual property, and confidential business data, is part of regulatory information management. Because any data breach or mishandling can result in legal action, damage to a company's reputation, and financial losses, data privacy and security concerns present a significant challenge.
Regulatory compliance challenges
Regulatory compliance requirements vary across industries and regions, making it challenging to develop a standardized regulatory information management solution that meets all specific requirements. Adapting to changing regulations and keeping up with compliance updates can be demanding and time-consuming.
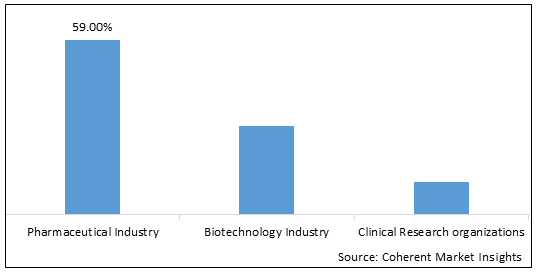
Figure 2. Global Regulatory Information Management Market, By End-use Industry
To learn more about this report, request a free sample copy
Global Regulatory Information Management Market Segmentation:
The global regulatory information management market report is segmented into Product Type and End Use Industry.
Regulatory information management software is offered with various features, which help organizations follow updated regulations. Software innovation and development have resulted in improved procedures for the efficient management of regulatory information. The software also guarantees a shorter turnaround time for any system issues or errors. Pharmaceutical companies are involved in the development, dissemination, and control of regulatory information throughout the product development cycle. Regulatory information management is an important solution for pharmaceutical companies as it effectively communicates regulations. Technology tools, products, and service platforms create opportunities for automating business practices resulting in optimum business output with rigid observance of the norms in the industry set by regulatory bodies and governments. In the pharmaceutical industry, regulatory information management software facilitates the robust application of planning, viewing, publishing, registration, and management of products throughout its life cycle. The software also allows effective compliant management of regulations and regulatory information. It has advantages such as the submission of plans, e-submission viewers, and product registration and tracking. The software enables tracking the periodic safety updated reports (PSUR) and real-time access to regulatory information management.
Global Regulatory Information Management Market: Key Developments
In February 2023, Aris Global LLC, a leading provider of life sciences software designed to automate core drug development functions with its end-to-end technology platform Life Sphere, launched Regulatory Information Management Solution Purpose-Built for Investigational-Stage Companies.
In October 2021, Calyx, one of the top ten pharmaceutical companies in the world, signed a contract to continue using the Calyx Regulatory Information Management (REGULATORY INFORMATION MANAGEMENT) system for crucial submissions of clinical trial data to international regulators through 2026.
In May 2023, The UK Medicines and Healthcare products Regulatory Agency (MHRA) issued new guidance for identifying and reporting adverse events involving Software as a Medical Device (SaMD) under the vigilance system.
In May 2023, Amplexor Life Sciences joined Aris Global, creating the Most Comprehensive Regulatory SaaS Platform for the Industry
Global Regulatory Information Management Market: Key Companies Insights
The global regulatory information management market is highly competitive. This is attributed to the continuous launch of new technologies due to ongoing R&D and efforts by value chain participants. Moreover, key players are adopting various business growth strategies in order to expand their presence on a regional as well as global basis.
Some of the key players in the global regulatory information management market are Acuta, Llc, Parexel, Computer Sciences Corp (CSC), Aris Global LLC, Virtify, Ennov, Amplexor, Samarind Ltd, Dovel Technologies, Inc., and Informa.
*Definition: The process of managing all regulatory information is known as regulatory information management. This information has historically been stored in a variety of systems, including Excel documents, file shares, cloud systems, and pen-and-paper repositories.
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients