The small molecule injectable drugs market is estimated to be valued at USD 234.74 Bn in 2025 and is expected to reach USD Bn 410.46 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The small molecule injectable drugs market growth can be attributed to rising prevalence of chronic diseases and increasing demand for pain management and anti-infective injectable drugs for treatment. Additionally, growing geriatric population who are more prone to developing chronic conditions is further supporting the growth of small molecule injectable drugs industry. Manufacturers are continually focusing on developing enhanced delivery and drug solutions to offer improved patient outcomes. Furthermore, increasing research and development activities for designing advanced injectable drugs will likely provide new opportunities for the market growth.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
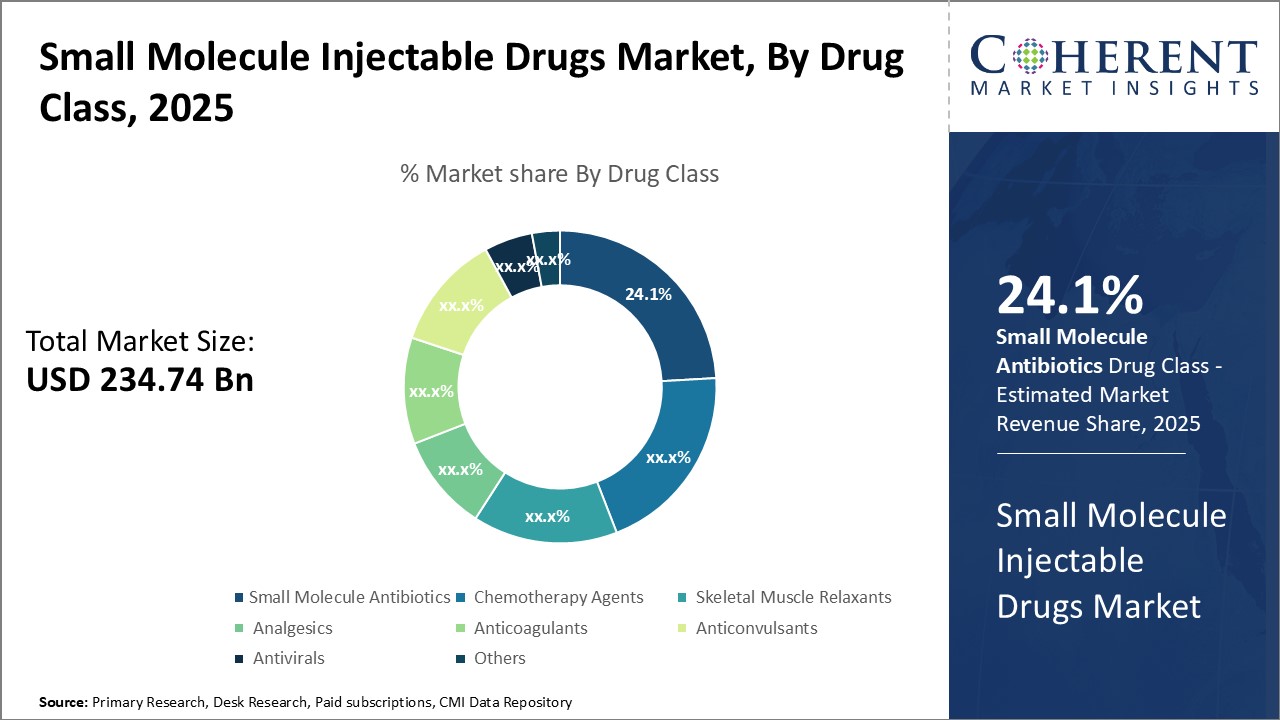
Insights by Drug Class: Rising prevalence of infectious diseases drives small molecule antibiotics demand
In terms of drug class, small molecule antibiotics segment is expected to hold the highest market share of 24.1% in 2025, driven by the rising prevalence of infectious diseases worldwide. The growing resistance to existing antibiotics is prompting significant research for novel anti-infective drugs. Additionally, the complexity of surgical procedures increases the risk of healthcare-associated infections, further driving the segment growth.
Insights by Indication: Rising cancer incidence fuels the oncology segment growth
Based on indication, the oncology segment is expected to hold the highest market share of 49.9% in 2025, driven by the rising global cancer incidence. Unhealthy lifestyles and physical inactivity are significant contributors to this cancer epidemic. Increased medical awareness of early detection and screening programs has led to higher diagnosis rates, boosting demand for targeted small molecule chemotherapeutics administered via the parenteral route.
Insights By Mode of Delivery: Advantages over oral route drives the intravenous segment growth
In terms of mode of delivery, intravenous segment is estimated to hold highest market share of 49.9% in 2025, due to its advantages over oral administration. It ensures accurate dosing, provides a quick onset of action, and improves bioavailability for drugs with narrow therapeutic indices. This makes IV the preferred choice for time-critical treatments in areas like acute pain management, infections, and cardiovascular emergencies.
Need a Different Region or Segment? Download Free Sample
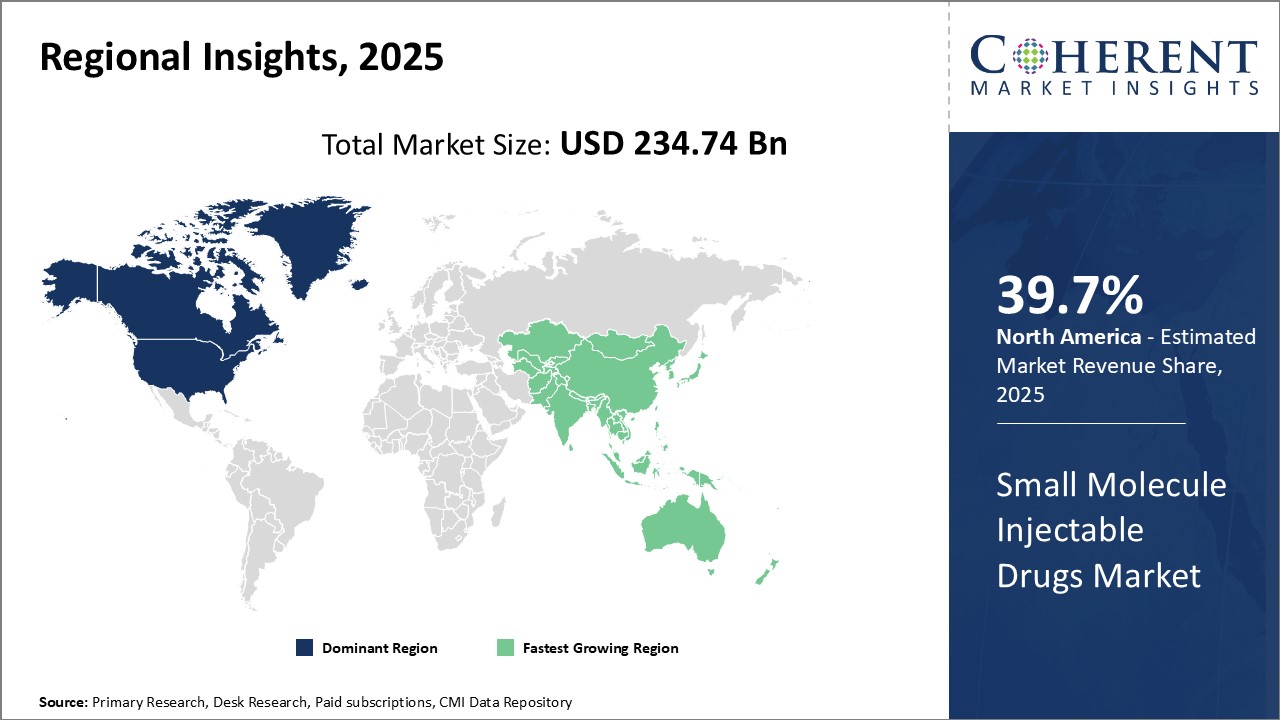
Dominating Region: North America
North America is expected to account for the greatest revenue share of 39.7% in 2025. North America’s dominance in the small molecule injectable drugs sector can be attributed to strong healthcare infrastructure and presence of leading pharmaceutical companies.
Fastest-Growing Region: Asia Pacific
The Asia Pacific region exhibits the fastest growth with 20% market share in 2025, due to rising healthcare expenditure, large patient pool, and increasing focus of major players on emerging countries are driving the market in the region.
Small Molecule Injectable Drugs Market Outlook for Key Countries
U.S.’ high adoption of novel drugs and therapies
The U.S. small molecule injectable drugs industry is characterized by a high adoption of novel therapies, with key players like Pfizer, Inc. and Merck & Co., Inc. maintaining a strong presence through continuous innovation. Their commitment to research and development enables them to introduce advanced treatments that address evolving patient needs and enhance therapeutic outcomes in a competitive landscape.
China's strong domestic generics industry
China's small molecule injectable drugs industry is experiencing robust growth, driven by a strong domestic generics industry and the increasing focus of international brands on local opportunities. Collaborations between local pharmaceutical companies and global players further strengthen their market position, enhancing innovation and expanding access to effective treatments in a rapidly evolving healthcare landscape.
Japan's Leadership in Small Molecule Injectable Drugs Portfolio
Japan continues to lead the Asia Pacific small molecule injectable drugs market through its universal healthcare coverage and emphasis on specialization. Companies like Daiichi Sankyo Company address a wide range of disease conditions with their extensive product portfolio, ensuring access to effective treatments while maintaining high standards in patient care and innovation within the healthcare system.
India’s government initiatives to boost local production of finished dosages
India's small molecule injectable drugs sector is expanding, supported by low-cost manufacturing capabilities and government initiatives to boost local production of finished dosages. Indian companies, such as Cipla, are actively exporting generic drugs globally, capitalizing on the country's competitive manufacturing environment to enhance their market presence and meet international demand.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Key Market Players
Emerging Startups in the Small Molecule Injectable Drugs Market
Key Takeaways from Analyst
Small Molecule Injectable Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 234.74 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 410.46 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
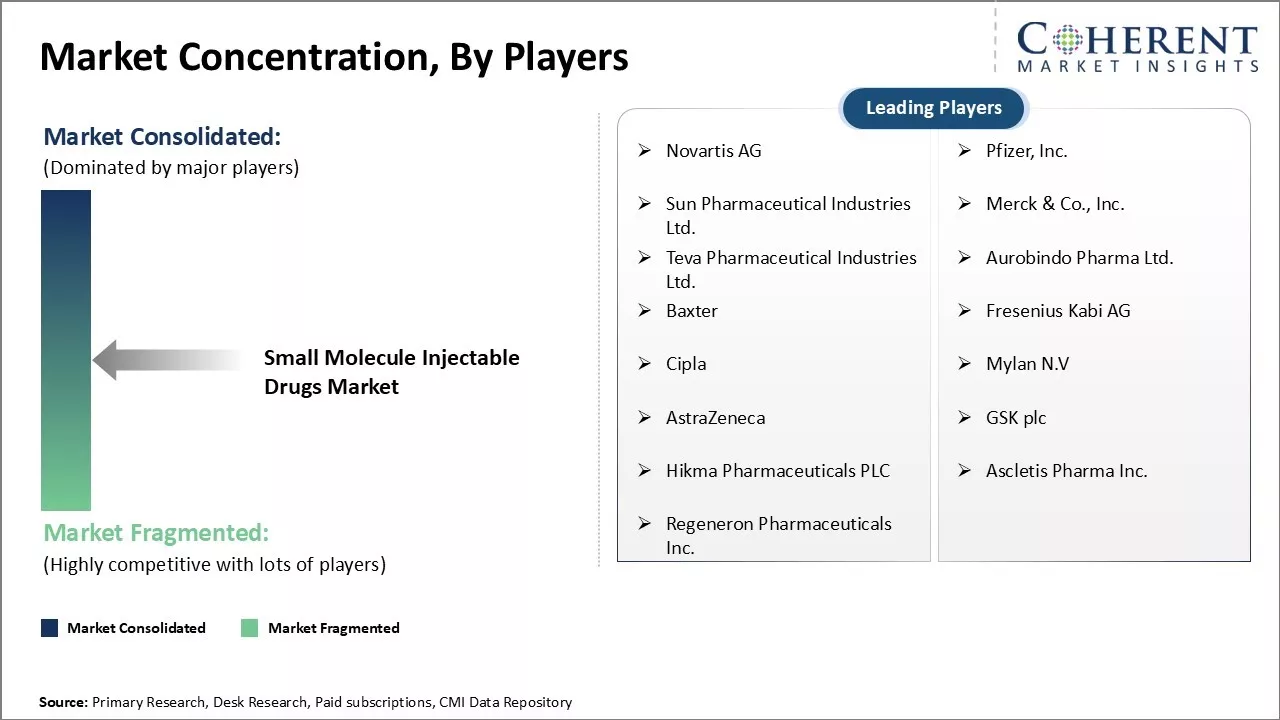
Novartis AG, Pfizer, Inc., Sun Pharmaceutical Industries Ltd., Merck & Co., Inc., Teva Pharmaceutical Industries Ltd., Aurobindo Pharma Ltd., Baxter, Fresenius Kabi AG, Cipla, Mylan N.V, AstraZeneca, GSK plc, Hikma Pharmaceuticals PLC, Ascletis Pharma Inc., and Regeneron Pharmaceuticals Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
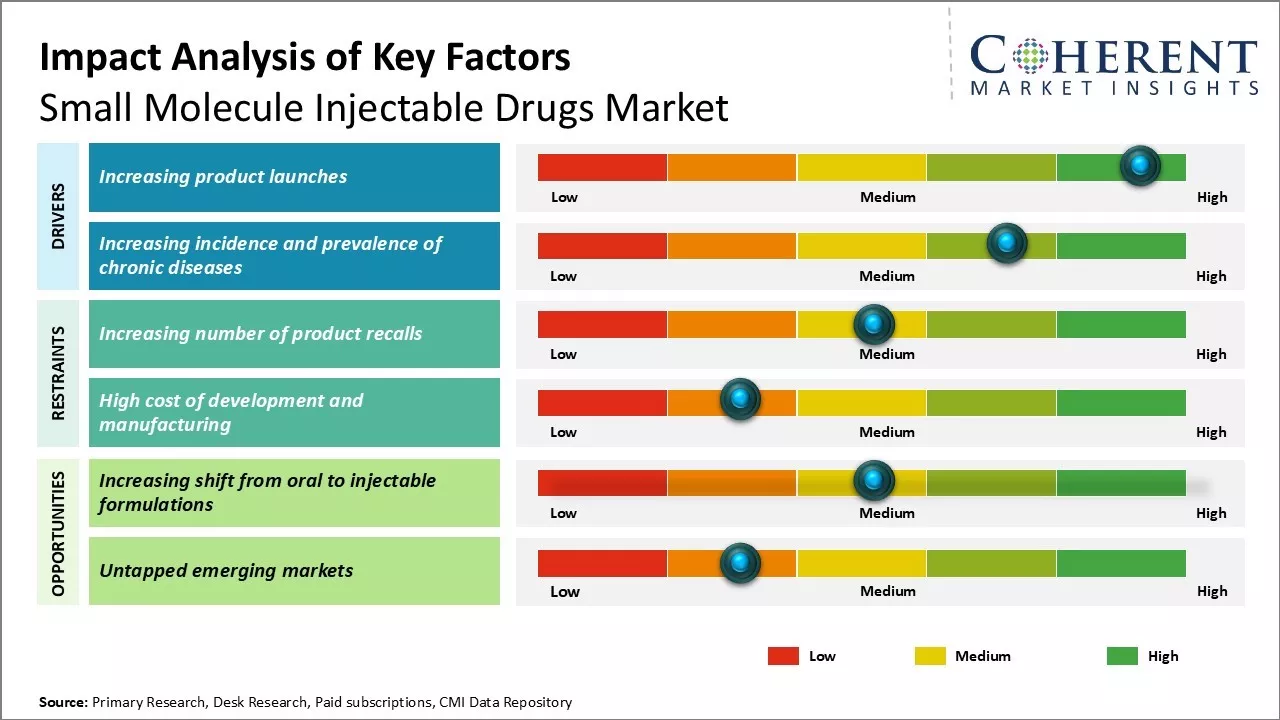
Market Driver - Increasing Product Launches
The small molecule injectable drugs market has been witnessing several product launches in the recent years which has been instrumental in driving the market growth. For instance, on June 24, 2024, Teva Pharmaceutical Industries Ltd, a pharmaceutical company, announced the launch of an authorized generic of Victoza 1 (liraglutide injection 1.8mg), in the U.S. It is indicated to lower blood sugar in adults and children with type 2 diabetes mellitus.
Market Challenge - Increasing number of product recalls
The small molecule injectable drugs market is facing significant challenges due to a rising number of product recalls. Regulatory authorities worldwide have adopted a zero-tolerance stance on manufacturing issues and contamination, prompting companies to initiate nationwide recalls even for minor quality concerns. This trend has surged over the past five years, disrupting supply chains, damaging company reputations, and burdening drug manufacturers with substantial recall costs and regulatory penalties.
Market Opportunity - Increasing shift from oral to injectable formulations
The small molecule injectable drugs market is expanding due to a shift from oral to injectable formulations. Injectable drugs offer superior bioavailability, ensuring 100% absorption into the bloodstream, which accelerates therapeutic effects. This makes them ideal for urgent treatments in emergency and critical care. Additionally, self-injection devices enhance convenience, creating opportunities in oncology, pain management, anti-infectives, and cardiovascular emergencies. Manufacturers are focusing on new product launches and advanced delivery systems to improve patient adherence.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients