Global Transthyretin Amyloidosis Treatment Market – Insights
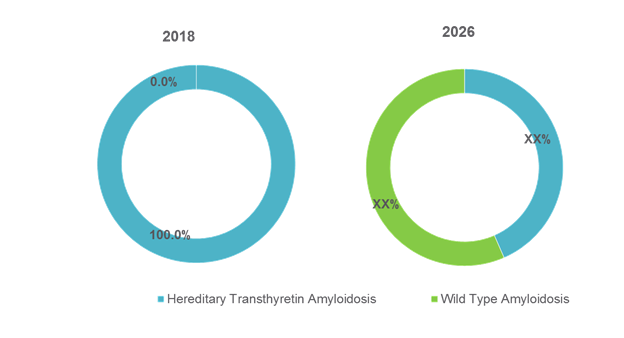
Hereditary transthyretin amyloidosis (hATTR) and wild type transthyretin amyloidosis are two main types of transthyretin amyloidosis. hATTR is further classified into Familial Amyloid Polyneuropathy (FAP) and Familial Amyloid Cardiomyopathy (FAC). Wild type variant (ATTRwt) predominantly affects the heart.
Transthyretin amyloidosis is a result of transthyretin produced by the liver and forms dimers, followed by monomers. Monomers aggregate to form amyloid fibrils, which are deposited in organs such as heart, nervous system, gastrointestinal tract, and kidneys. FAP is a subtype of hereditary transthyretin amyloidosis and the most common type of FAP is caused by the Val30Met variant of Transthyretin (TTR).
In familial amyloid polyneuropathy, the symptoms are first detected after the patient crosses 30 years of age, however, it can also be detected as early as 20 years or as late as 80 years of age. Symptoms are divided depending on the location such as peripheral neuropathy and autonomic neuropathy. Symptoms may worsen in case excess amyloid protein starts to collect in the nerves.
Introduction of novel therapy for the treatment of transthyretin amyloidosis is expected to significantly drive the market growth
Key players in the market are focused on approval and launch of novel therapies for treatment of transthyretin amyloidosis. This in turn is expected to propel growth of the global transthyretin amyloidosis treatment market over the forecast period.
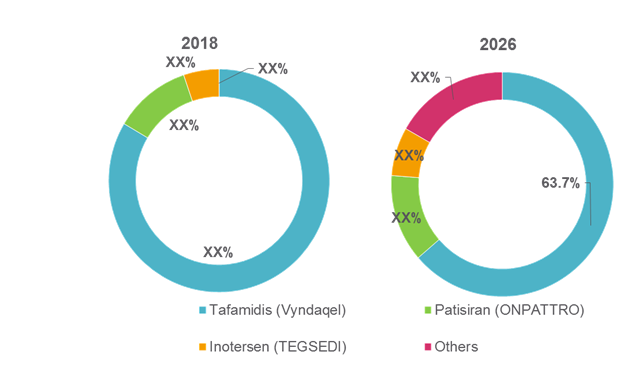
For instance, in August 2018, Alnylam Pharmaceuticals, Inc. received the U.S. Food and Drug Administration (FDA) approval for its ONPATTRO (patisiran) lipid complex injection— a RNA interference (RNAi) therapeutic— indicated for the treatment of polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
In July 2018, Ionis Pharmaceuticals, Inc. and Akcea Therapeutics, Inc. received marketing authorization approval for its drug TEGSEDI (inotersen) from the European Commission (EC) for the treatment of stage 1 or stage 2 polyneuropathy in adult patients with hereditary transthyretin amyloidosis.
The global transthyretin amyloidosis treatment market size is expected to be valued at US$ 35.8 million in 2018 and is expected to witness a robust CAGR of 55.4% over the forecast period (2018–2026).
Figure 1. Global Transthyretin Amyloidosis Treatment Market Share (%), by Drug Type, 2018 and 2026
To learn more about this report, Download Free Sample
Robust pipeline of transthyretin amyloidosis treatment drugs is expected to boost the market growth over the forecast period
Major players in the market have novel drugs in the pipeline, which are in late-stage clinical trials and are expected to receive approval in the near future. For instance, Eidos Therapeutics, Inc. is developing AG10— an orally-administered, small molecule designed to potently and selectively stabilize tetrameric TTR, thereby interfering with events that give rise to ATTR. The drug is currently in Phase 2 clinical trial.
Akcea Therapeutics Inc. is co-developing AKCEA-TTR- LRx with Ionis Pharmaceuticals to inhibit the production of transthyretin. Akcea Therapeutics Inc. is focused on developing AKCEA-TTR-LRx for patients with both hereditary and wild type form of the disease. AKCEA-TTR-LRx is planning to enter clinical development in 2018.
Figure 2. Global Transthyretin Amyloidosis Treatment Market Share (%), by Disease Type, 2018 and 2026
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2017)
Key players in the market are focused on strategic partnerships in order to enhance their market share. For instance, in August 2018, Alnylam Pharmaceuticals, Inc. partnered with Orsini Healthcare, a specialty pharmacy as distributor of ONPATTRO (patisiran) lipid complex injection.
However, high costs of recently launched drugs is expected to hinder growth of the market. For instance, according to Alnylam Pharmaceutical, Inc., the cost of Patisiran is around US$ 450,000, annually before insurance. Another drug, Inotersen manufactured by Ionis Pharmaceuticals in partnership with Akcea Therapeutics, received approval for hereditary Transthyretin Amyloidosis Polyneuropathy indication and the drug costs US$ 450,000, annually. High cost of drugs may result in lower adoption even in developed economies such as the U.S., thereby restraining growth of the market.
Key players operating in the global transthyretin amyloidosis treatment market include, Alnylam Pharmaceuticals, Inc., Pfizer, Inc., Prothena Corporation Plc., GlaxoSmithKline Plc., Ionis Pharmaceuticals, Inc., Eidos Therapeutics, and SOM Innovation Biotech, S.L.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients