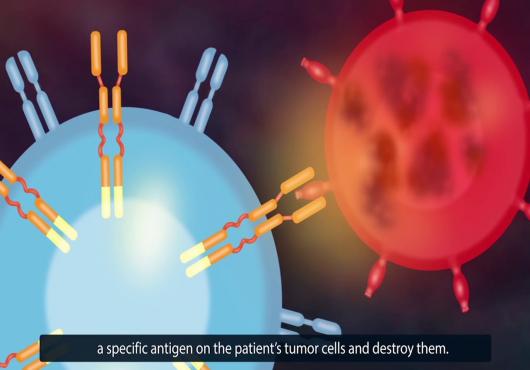
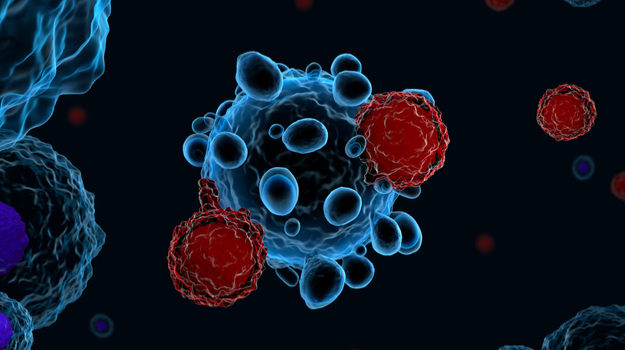
Cancer immunotherapy aims to eradicate tumors without the toxicity associated with conventional cancer treatments. Tumors suppress immunity by evading host immune-response. Strengthening immunity is the most appropriate treatment to thwart nasty cancerous tumors. Immunotherapy focuses on strengthening the body’s natural immune system to fight cancer. In CAR T Cell therapy, T lymphocytes (T cells) are collected through blood samples and genetically engineered in laboratories to produce Chimeric Antigen Receptor (CAR) on their surface. CAR are proteins that allow T cells to recognize an antigen on target tumor cells. After millions of CAR T-cells are generated, they are again infused into the patient’s body, which then acts as attacker cells to accurately detect and kill cancer cells. As they continue to remain in the body, they prevent cancer-reoccurrence, thus providing a long-term solution from the malady.
The first clinical trials of CAR T cells began in 1996 on patients suffering from ovarian cancer and the results showed limited efficacy. However, over the last two decades, significant advancements in molecular biology and immunology have shown significant success in clinical trials and in overcoming challenges.
Car T cell therapy researches have been fairly successful in Acute Lymphoblastic Leukaemia (ALL), multiple myeloma, neuroblastoma, pancreatic cancer, breast cancer, and other blood-related cancers especially in children. As per statistics released by the Leukemia and Lymphoma Society in 2016, a blood cancer patient is diagnosed roughly every 3 minutes. Moreover, 10.2% of diagnosed cancer cases in the U.S. suffer from either leukemia, lymphoma, or myeloma. Increasing the incidence of cancer in the U.S. and Europe and rising expectations for a reliable smooth treatment will generate a lucrative revenue for the CARTs market. Advanced research techniques, a strong economy, robust infrastructure further enhance the potential of these countries as pockets of massive growth in the near future. The Asia Pacific, spearheaded by the advanced technological research infrastructure in Japan is expected to position the region as a lucrative market post–commercialization of the product. However, the low prevalence of cancer and the high cost of the therapy are setbacks for market growth in these regions.
Prominent companies pioneering in commercializing CAR T cell therapy are Novartis International AG, Juno Therapeutics, Kite Pharma Inc, Bellicum Pharmaceuticals Inc, Colgene Corporation and many more.
Product launches in the pipeline expected to boost the market
Currently, CAR T cells therapy is available only to patients on the clinical trial, though with clinical evaluation growing exponentially two companies Novartis International AG and Kite Pharma Inc are in plans to register their respective products, CTL019(anti-CD19) targeting lymphoblastic leukaemia in paediatrics and Kte-C19(anti-CD19) with B-cell lymphoma, in 2017. Juno Therapeutics hit a roadblock in launching JCAR015, a product targeting adult patients due to the death of five patients under clinical trial due to therapy-induced cerebral edema. Recently, Novartis shut its cell and gene therapy unit, apparently an unexpected move.
Side effects restraining the growth
Major side effects encountered are cytokine-release syndrome: excess cytokine-release after T-cell activation, causes delirium, low blood pressure, and poor lung oxygenation; B-cell aplasia where normal B-cells are destroyed by antigens though long-term effects have not been found; Tumor lysis syndrome, a metabolic complication arising due to breakdown of dying cells but can be controlled by supportive therapy. Potential solutions such as the introduction of suicide-gene T-cells causing the death of the engineered T-cells after the required period help negate side effects. Manufacturing-infrastructure, personalized- approach of the therapy, side-effect handling: all these factors attach an exorbitant price tag to this treatment. Affordability, a big limitation but longevity of the treatment and broader applicability to all age-groups are significant add-ons.
Despite these restraints, CAR T cell therapy is a promising option for relapsed patients after intensive chemotherapy and stem cell transplant. The depleted immune system is a drastic problem raising mortality in cancer patients hence immunity-boosting CARTs is a boon to patients as a successful application of CAR T cell therapy on specifically targeted tumor cells would minimize toxicity risks. Once, initial hurdles are surpassed, this immunity-enhancing autologous therapy is sure to make waves and rope in the menace of cancer lurking on mankind.
To know more about the latest market trends and insights, read the report titled "Global CAR T Cell Therapy Market, By Targeted Antigen(CD 19, CD 20, GD2, CD22, CD30, CD33, HER1, HER2, Meso, EGFRvlll), Therapeutic Application(Acute Lymphocytic, Leukemia, Chronic Lymphocytic Leukemia, Non-Hodgkin Leukemia, Multiple Myeloma, Pancreatic Cancer, Neuroblastoma, Breast Cancer, Acute Myeloid Leukemia, Hepatocellular Carcinoma, Colorectal Cancer), and Geography - Trends, Analysis, and Forecast till 2028", published by Coherent Market Insights. Click on the link below to access the report –
https://www.coherentmarketinsights.com/market-insight/car-t-cell-therapy-market-102






