The Bioresorbable Vascular Scaffold Market is estimated to be valued at USD 480 Mn in 2026 and is expected to reach USD 960 Mn by 2033, growing at a compound annual growth The rate (CAGR) of 8.5% from 2026 to 2033.
The Bioresorbable Vascular Scaffold Market is advancing significantly, driven by its expanding role in interventional cardiology and the global shift toward avascular reparative therapy. These scaffolds provide temporary mechanical support to the vessel wall before the body naturally reabsorbs them, which distinguishes them from traditional permanent metallic stents. This approach drove market growth because clinicians aim to reduce long-term complications such as late stent thrombosis and permanent vessel restriction. This technology provides a promising alternative to patients who suffer from coronary and peripheral artery diseases by allowing the artery to retain its natural flexibility and function.
|
Current Event |
Description and the Impact |
|
Technological Advancements & Innovation |
|
|
Competitive Landscape & Market Consolidation |
|
|
Clinical Evidence & Physician Adoption |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of material, the polymer‑based BVS segment contributes the highest share of 76.20% in the market in 2026 driven by the unique ability of polymers to vanish completely after restoring vessel health. These materials facilitate the return of natural arterial movement, or vasomotion, because they do not permanently cage the blood vessel like traditional metal stents. The medical device companies continue to refine polymer chemistry to create thinner struts, which substantially improve blood flow and reduce the risk of long-term blood clots. Thus, this temporary support strategy provides the benefits of stenting without leaving a permanent foreign body in the avascular system.
In terms of product type, the synthetic segment contributes the highest share of 88.60% in 2026 of the market. The synthetic materials provide engineers with unparalleled control over chemical and physical properties. The manufacturers are utilizing synthetic polymers like Polylactic Acid (PLA) to precisely program the dissolution rate of the scaffold, thus ensuring the device provides structural support only during the vital vessel healing window. These materials provide superior reliability and lower production costs compared to natural alternatives, making them the primary choice for high-volume medical manufacturing. As a result, the synthetic category is maintaining its leading position by delivering predictable clinical outcomes and streamlined regulatory approval processes.
In terms of application, the coronary artery stents segment contributes the highest share of 67.30% in 2026 of the market. This growth is owing to the urgent global need to treat coronary artery disease (CAD). The rising rates of obesity, diabetes, and aging populations drive massive demand for effective heart interventions that preserve future surgical options such as bypass grafting. Surgeons are preferring bioresorbable scaffolds for these applications because they stabilize the heart's arteries during the critical recovery phase before dissolving away. This approach is attractive to both patients and clinicians, as it mitigates chronic inflammatory responses and lowers the risk of late-stage complications linked to permanent metallic implants.
To learn more about this report, Download Free Sample
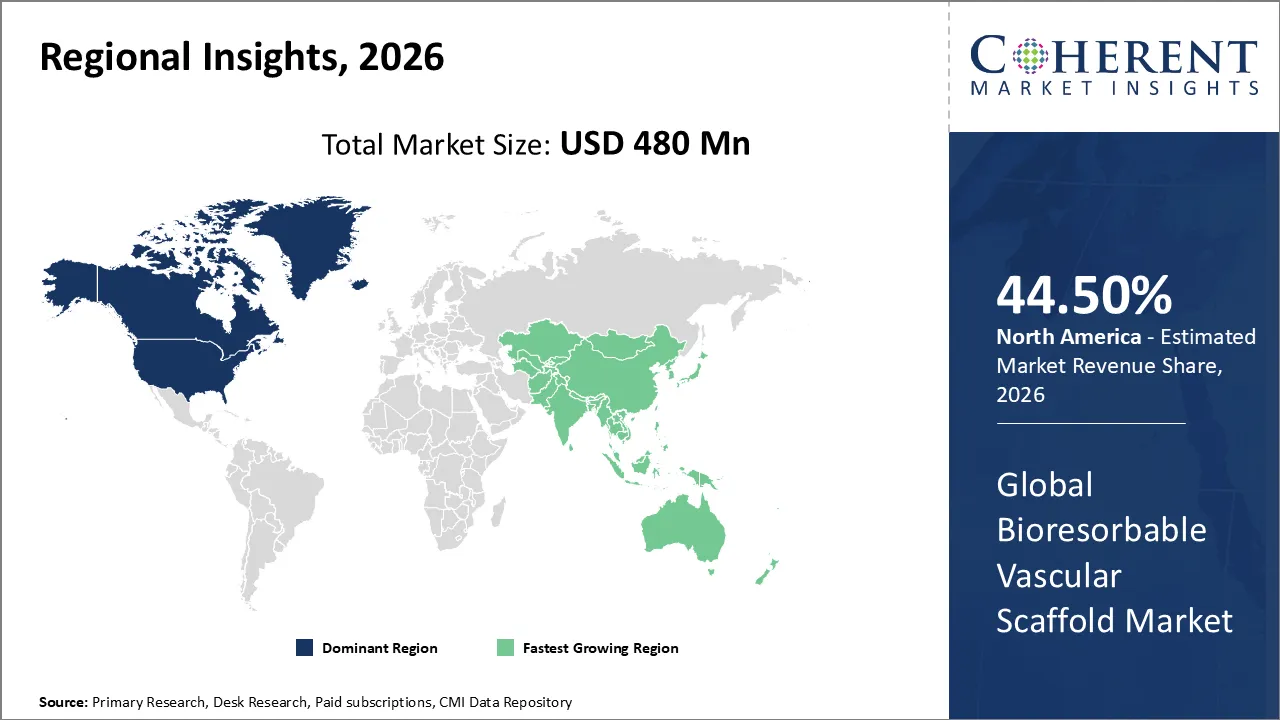
North America has remained the dominant region with 44.50% in 2026 of the global Bioresorbable Vascular Scaffold Market over the past decade, contributing nearly half of all global revenue. The major companies like Abbott and Boston Scientific, with highly advanced healthcare infrastructures and intense research and development, propel the growing demand in the market. The region has benefitted from early access to next-generation technologies, such as the FDA’s 2024 approval of the Esprit BTK system, which has stirred new interest in scaffold applications. The high healthcare expenditure and robust reimbursement frameworks also facilitate the widespread adoption of these procedure devices. The clinician preference for leave-nothing-behind coronary interventions is likely to contribute to sustained growth of the North American market throughout the forecast period. Providers in the region favor minimally invasive surgical approaches to effectively address widespread cardiovascular conditions and improve long-term clinical outcomes.
The Asia Pacific region represents the fastest-growing market for bioresorbable scaffolds. A massive patient pool and the rising incidence of diabetes and hypertension in China and India are fueling this rapid surge. Local players, such as Meril Life Sciences, are speeding up the growth by developing cost-effective solutions that challenge the pricing of traditional Western alternatives. The governments in the region are increasing healthcare investments and streamlining regulatory pathways to modernize cardiac care for their citizens. The rising medical tourism and greater awareness of minimally invasive procedures among the expanding middle class are enabling Asia Pacific to quickly close the gap with Western markets. As a result, the region is transitioning from a late adopter into a primary global hub for scaffold manufacturing and clinical application.
The US currently commands the largest share of the global bioresorbable vascular scaffold market due to its advanced healthcare infrastructure and high research investment. The market continues to benefit from recent regulatory approvals for second-generation scaffolds that address previous mechanical limitations. The technological advances are promoting a broader adoption of fully resorbable treatment methods among US cardiologists for both coronary and peripheral artery diseases. In addition, robust reimbursement frameworks and the high prevalence of cardiovascular conditions sustain the country’s position as the primary revenue generator. The leading medical institutions are also integrating advanced imaging technologies to ensure more precise placement and better long-term monitoring of these dissolving devices.
China represents the fastest-growing segment of the bioresorbable vascular scaffold market as the country prioritizes modernization in cardiac care. This growth follows aggressive government initiatives to reduce dependence on expensive imported medical devices in favor of domestic production. The local innovators now produce high-quality scaffolds that cater specifically to the country’s massive and aging patient population. In addition, rising healthcare investments and streamlined regulatory pathways facilitate the rapid adoption of these minimally invasive therapies across Chinese hospitals. Consequently, China is quickly evolving into a major global hub for scaffold manufacturing and clinical application.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 480 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.5% | 2033 Value Projection: | USD 960 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AngioDynamics, Inc., C. R. Bard, Inc., Teleflex Incorporated, B. Braun Melsungen AG, Medtronic Plc, Vygon Ltd., Cook Medical Inc., Argon Medical Devices, Inc., ICU Medical, Inc., and Theragenics Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The rising prevalence of cardiovascular diseases, particularly coronary artery disease, serves as the primary catalyst for the global expansion of the bioresorbable vascular scaffold market. Sedentary lifestyles and aging populations increase the incidence of arterial blockages, forcing healthcare providers to seek more effective long-term interventions for younger and high-risk patients. The scaffolds provide temporary structural support and are fully reabsorbed into the vessel wall, in contrast to traditional metallic stents that remain permanently implanted. This process allows the treated artery to regain its natural vasomotion and flexibility, which substantially reduces the risk of long-term complications like chronic inflammation or permanent vessel caging. As a result, a massive invasive armament drives manufacturers to speed up the production of these advanced devices to meet the growing clinical demand.
For instance, in May 2024, R3 Vascular Inc., a medical device company specializing in the production and sale of innovative bioresorbable scaffolds for the treatment of peripheral artery disease (PAD), has successfully completed its Series B investment round, raising USD 87 million.
The bioresorbable vascular scaffold market is demonstrating structurally improving adoption dynamics, supported by expanding clinical evidence, technological refinement, and a growing preference for temporary vessel support solutions in interventional cardiology. Industry data indicate a clear rebound in procedural utilization over recent years, with scaffold deployment volumes increasing across major cardiac care centers as newer-generation devices address earlier safety and performance concerns.
Coronary artery disease remains the dominant application area, accounting for the majority of scaffold usage due to high angioplasty procedure volumes globally. Peripheral vascular applications are also gaining traction, particularly in below-the-knee interventions, supported by targeted clinical trials and improved deliverability profiles. Hospitals continue to represent the primary end-use setting, reflecting the need for advanced catheterization laboratories and specialized interventional expertise.
Technological innovation is a key market enabler. A growing proportion of development pipelines now emphasize magnesium-based and advanced polymer scaffolds, which offer faster and more predictable resorption timelines compared to first-generation designs. Drug-eluting configurations dominate new product launches, with antiproliferative agents integrated to reduce restenosis and improve vessel healing outcomes. Clinical datasets increasingly report improved lumen restoration and reduced long-term foreign-body presence, reinforcing clinician confidence.
Regionally, North America leads adoption due to strong clinical research activity and established interventional cardiology infrastructure, while Asia Pacific is emerging as a high-growth region driven by rising cardiovascular procedure volumes and expanding cath lab capacity. Overall, the market outlook remains positive, supported by data-driven innovation and widening clinical acceptance.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients