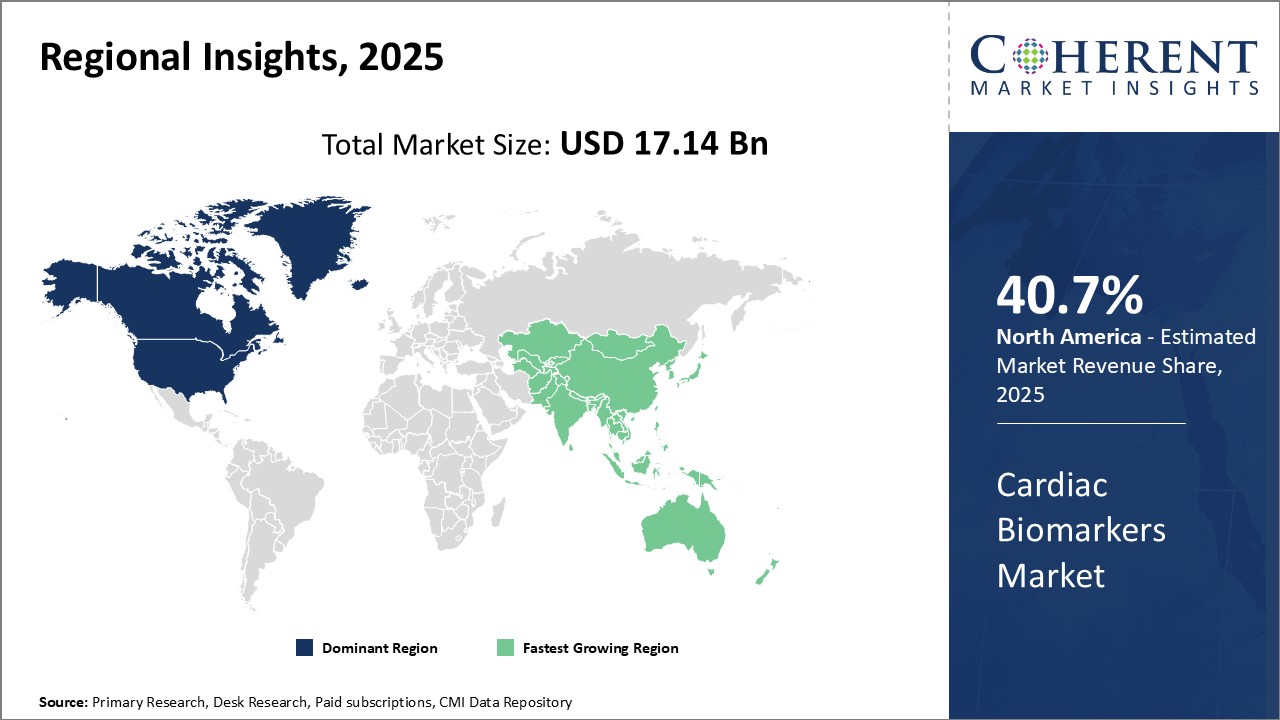
The global cardiac biomarkers market is estimated to be valued at USD 17.14 Bn in 2025 and is expected to reach USD 41.62 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 13.5% from 2025 to 2032.
The increasing prevalence of cardiovascular diseases and the growing demand for point-of-care testing are the major factors contributing to the market growth. The market is witnessing various trends such as increased adoption of high sensitivity troponin tests and launch of novel cardiac biomarkers by key players.
Moreover, rising investments by public and private players to develop innovative biomarkers with high sensitivity and specificity for accurate detection of acute myocardial infarction and other heart conditions are expected to offer lucrative opportunities for the market players over the forecast period.
To learn more about this report, Download Free Sample
To learn more about this report, Download Free Sample
AI is revolutionizing cardiac diagnostics, especially in biomarkers, enhancing accuracy, efficiency, and predictive capabilities
|
Factor |
FDA (U.S.) |
EMA (EU) |
Emerging Markets |
|
Regulatory Framework |
CDRH + biomarker qualification |
IVDR + notified bodies + EMA opinion |
IVD regs & local body approval |
|
Key Approval Pathway |
PMA / 510(k); IDE for trials |
CE-marking (Class C/D), centralized MA |
Local registration/licensing |
|
Timeline & Cost |
~2–3 years, expensive R&D |
1–2 years, compliance team needed |
Varies widely |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
|
Current Events |
Description and its impact |
|
Technological Advancements in Biomarker Detection (Nano-Level and Technology Events) |
|
|
Global Rise in Cardiovascular Diseases (Macro-Level Healthcare Trend) |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The cardiac biomarkers market is moving towards specialized, high-potential applications that are currently underutilized.
|
Category |
Type |
Price Range (USD) |
|
Biomarker Class |
Troponin Tests |
15–50/test |
|
High-sensitivity Troponin (hs-TnT) |
25–75/test |
|
|
Conventional Troponin Assays |
15–35/test |
|
|
BNP/NT-proBNP |
20–60/test |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
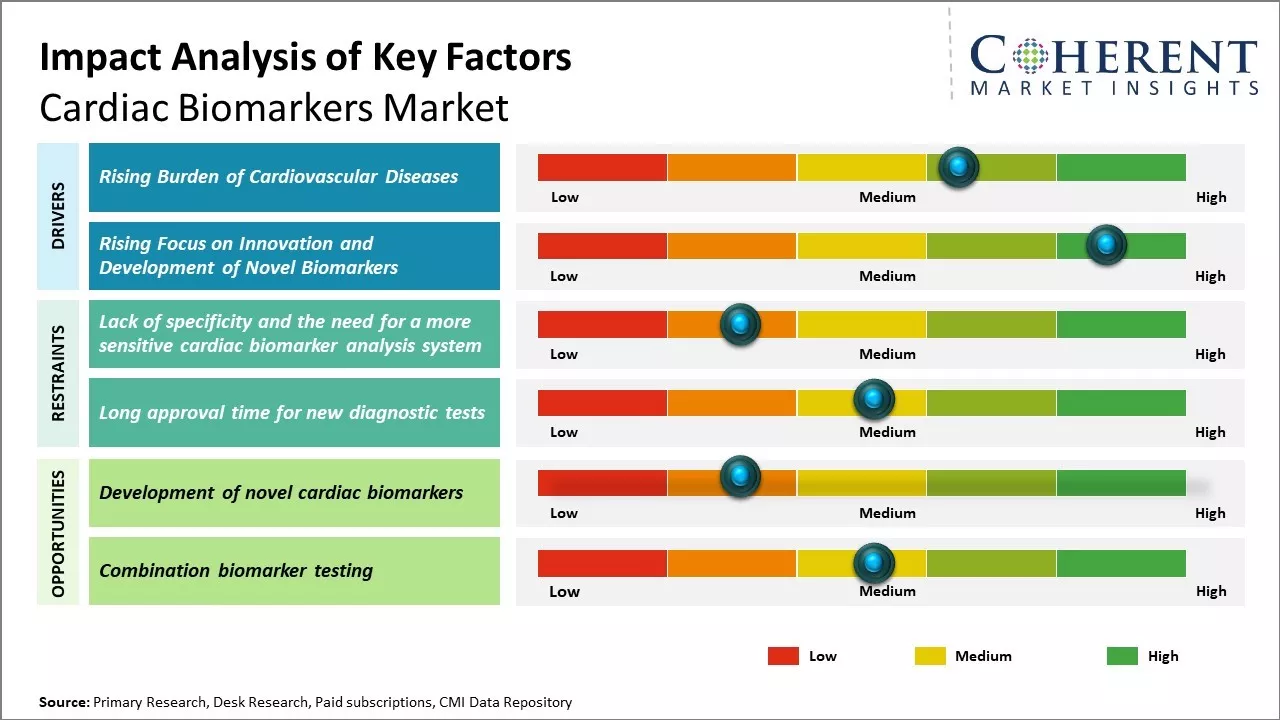
The increasing prevalence of cardiovascular diseases is one of the major drivers of growth in the cardiac biomarkers market. According to the World Health Organization, cardiovascular diseases account for over 17 million deaths each year, which is estimated to grow to over 23.6 million by 2030.
The key factors responsible for the rising cardiovascular disease burden include rapidly aging populations, increasing adoption of sedentary lifestyles and unhealthy diets, rising stress levels, smoking, and a growing prevalence of conditions like obesity and diabetes.
The growing focus of leading industry players and research institutions on innovation and development of novel cardiac biomarkers is another major factor fueling the growth of this market. Currently available biomarker tests only facilitate the detection of myocardial necrosis and injury.
However, clinicians also require biomarkers that can provide accurate information on other aspects such as myocardial stress, ischemia, remodeling, and other pre-damage states. The development of panels of multiple biomarkers is also gaining attention for facilitating comprehensive cardiac risk assessment.
The novel cardiac biomarkers are poised to revolutionize the global market by enabling early prediction of cardiac events, unlike current biomarkers such as Troponin and CK-MB, which only indicate injury after symptoms appear.
For instance, ST2 and Galectin-3 show promise in predicting heart failure exacerbations days in advance, offering a critical window for intervention. This shift towards predictive biomarkers is vital, considering cardiovascular diseases cause 17.9 million deaths annually worldwide, with nearly half occurring prematurely.
In terms of product type, Myocardial Muscle Creatine Kinase segment is expected to contribute the highest share of the market, accounting for 36.0% in 2025. This is owing to their higher sensitivity and specificity compared to other cardiac biomarkers.
Troponins, including Troponin T and Troponin I, are regulatory proteins that control the contractions of cardiac muscle. Even small amounts of Troponin released into the bloodstream can accurately indicate damage to the heart muscle. This makes Troponins the gold standard for the diagnosis of myocardial infarction as they can detect any heart muscle damage within 4-6 hours of the onset of chest pain. This increased diagnostic resolution provided by Troponins tests further expands their role in diagnosing conditions like unstable angina or detecting re-infarctions.
In terms of application, myocardial infarction segment is expected to contribute the highest share of the market, accounting for 36% in 2025. This is owing to its high prevalence and mortality rates globally.
The high incidence of myocardial infarction (MI) has boosted the demand for precise and early diagnosis of the condition. Since cardiac biomarkers play a crucial role in confirming suspected MIs by detecting heart muscle damage, their usage is concentrated majorly in MI diagnosis. Biomarkers like Troponins, CK-MB, and myoglobin allows clinicians to diagnose MI with higher accuracy than electrocardiograms or chest pain history alone. This supports clinicians in streamlining treatment decisions such as use of medications or angioplasty procedures.
In terms of location of testing, laboratory testing is expected to contribute the highest share of the market, accounting for 70.6% of the market share in 2025 owing to its ability to offer higher sensitivity cardiac biomarker tests and a broader menu of tests compared to point-of-care formats.
Furthermore, clinical laboratories have the capability to run a wide spectrum of cardiac biomarker tests including those for myoglobin, CK-MB, and different Troponin isoforms. This expansive cardiac test menu supports laboratories in precisely characterizing widespread cardiac damage. Additionally, clinical laboratories have well-established processes, skilled personnel, and robust quality assurances in place to deliver reliable test results.
To learn more about this report, Download Free Sample
North America has dominated the global cardiac biomarkers market and is expected to continue its dominance over the forecast period. The region is expected to account for 40.7% of the market share in 2025. This can be attributed to the high prevalence of cardiovascular diseases in the U.S. and Canada coupled with growing elderly population.
In addition, well-established healthcare infrastructure and rising healthcare spending in the region are supporting the demand for advanced cardiac diagnostic tests. The presence of leading cardiac biomarkers manufacturers and availability of reimbursement are further propelling the market growth.
Asia Pacific has emerged as the fastest growing region in the global cardiac biomarkers industry. Rapid economic development, growing medical tourism industry, and rising chronic disease burden are the key factors fueling the market in Asia Pacific countries.
Additionally, increasing awareness about cardiac condition diagnosis and management is boosting the adoption of cardiac biomarkers. Leading global manufacturers are shifting their focus towards the Asia Pacific region in view of opportunities for business expansion. This has enhanced the accessibility to advanced testing technologies in the region.
The U.S. cardiac biomarkers market is a rapidly growing sector, fueled by the rising burden of cardiovascular diseases and advancements in diagnostic technology. Troponin assays dominate, while point-of-care testing and AI integration are key trends driving market expansion, with major players actively innovating to meet growing demand for early and precise cardiac event detection.
India cardiac biomarkers market is seeing strong growth, driven by the increasing burden of heart diseases and a rising focus on early diagnosis. Troponin assays are particularly important due to their high accuracy. There's also a significant push towards point-of-care (POC) testing, which allows for quick results, critical for emergency care across diverse healthcare settings.
Cardiac Biomarkers Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 17.14 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.5% | 2032 Value Projection: | USD 41.62 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
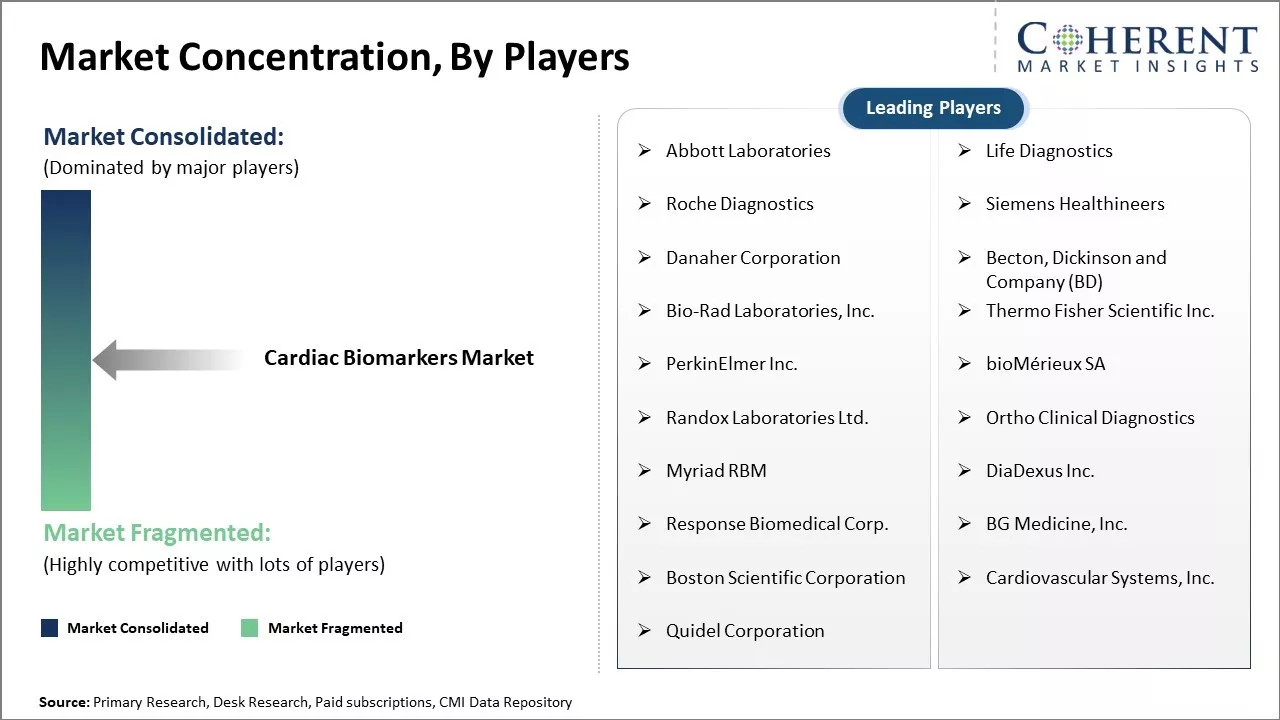
Abbott Laboratories, Life Diagnostics, Roche Diagnostics, Siemens Healthineers, Danaher Corporation, Becton, Dickinson and Company (BD), Bio-Rad Laboratories, Inc., Thermo Fisher Scientific Inc., PerkinElmer Inc., bioMérieux SA, Randox Laboratories Ltd., Ortho Clinical Diagnostics, Myriad RBM, DiaDexus Inc., Response Biomedical Corp., BG Medicine, Inc., Boston Scientific Corporation, Cardiovascular Systems, Inc., and Quidel Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: A cardiac biomarker refers to a protein-based substance that can be measured to analyze heart conditions for both prognostic and diagnostic purposes. These biomarkers are discharged into the bloodstream when the heart undergoes injury or stress, such as oxygen deprivation. Assessing cardiac biomarkers like cardiac troponin, creatinine kinase, and myoglobin, among others, aids in diagnosing various cardiovascular ailments like acute coronary syndrome (ACS) and cardiac ischemia.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients