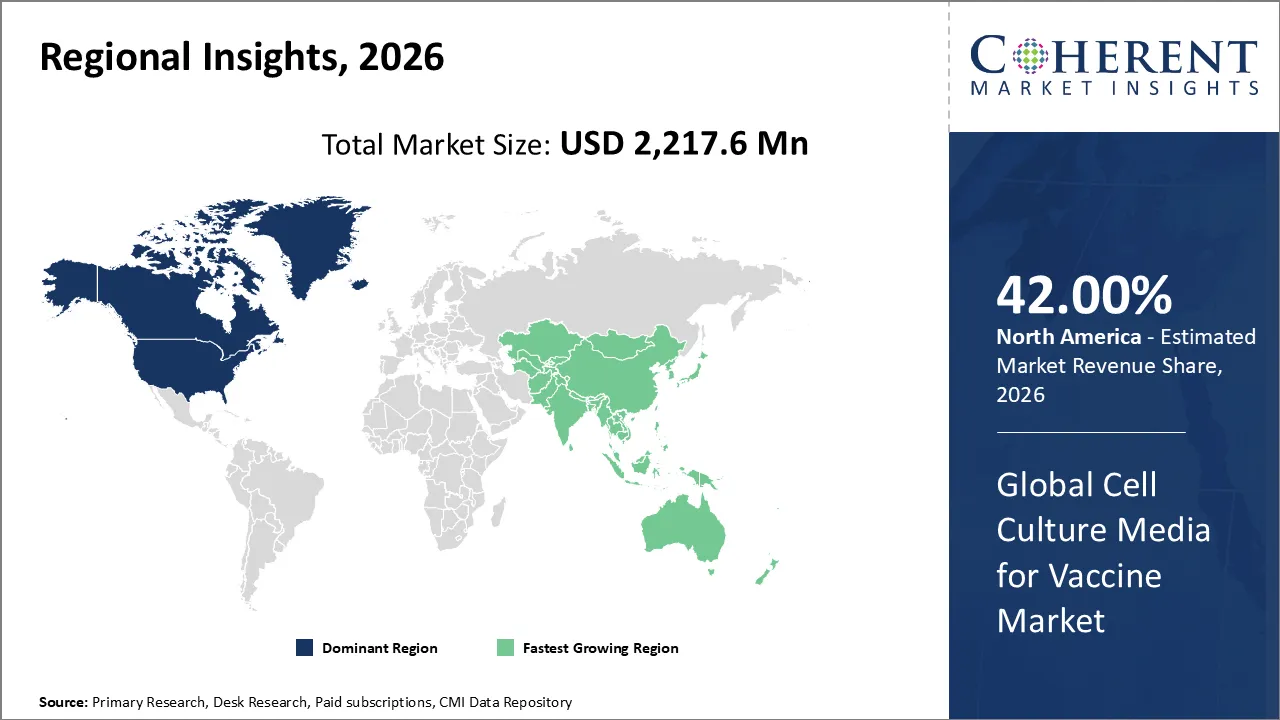
Cell culture media for vaccine market is estimated to be valued at USD 2,217.6 Mn in 2026 and is expected to reach USD 3,727.4 Mn in 2033, exhibiting a compound annual growth rate (CAGR) of 7.7% from 2026 to 2033.
Cell culture media for vaccine plays a major role in advanced healthcare, as it regulates the cell cycle. It is the growth medium, available in liquid or gel form, developed to support growth of microorganisms, cells, or small plants.
|
Current Event |
Description and its Impact |
|
Advanced Manufacturing Technology Adoption |
|
|
Next-Generation Vaccine Platform Development |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
|
Category |
Details |
|
Serum-Based Media |
~$300–500 per liter |
|
Serum-Free Media |
~$100–250 per liter |
|
Recombinant Albumin (e.g., Optibumin) |
~$1,000–2,000 per gram (high purity, GMP-grade) |
|
Contribution to Upstream Vaccine Production Costs |
15–25% of total upstream costs, making it a major cost driver |
|
Optimization Importance |
Critical for reducing batch-to-batch variability and ensuring cost-effective scalability |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of application, the human vaccine segment is expected to lead the market with 67.5% share in 2026, owing to global immunization programs, pandemic preparedness, and new technologies. The rise in the development of mRNA and recombinant vaccines, along with government-backed programs, has greatly increased the need for high-quality cell culture media made for human use, especially in new markets and areas with a lot of disease.
For instance, in December 2025, to help with vaccine research, SGS released a new RSV A strain challenge agent. This new idea helps clinical trials by giving researchers a reliable way to test how well a vaccine works. The development bolsters global initiatives against respiratory syncytial virus, fulfilling critical healthcare demands and expediting advancements in preventive medicine.
In terms of media type, the animal free/serum free cell culture segment is expected to hold 48.2% share of the market in 2026, due to more rising demand for vaccines that are made in a way that is ethical, consistent, and free of contamination. These formulations remove animal-derived components, which meets regulatory standards and makes bioprocessing easier to scale. Pharmaceutical and biotech companies prefer them because they work well with modern vaccine platforms like mRNA and recombinant technologies.
For instance, in November 2025, Evonik and InVitria announced they are working together to make animal-free recombinant albumin more widely available around the world. This will help biopharma innovation. The goal of the partnership is to make vaccine and drug development better by providing safer, more sustainable alternatives to traditional materials, making supply chains stronger, and speeding up progress in cell culture and biomanufacturing around the world.
To learn more about this report, Download Free Sample
North America is expected to dominate the cell culture media for vaccine market with 42% share in 2026, driven by its advanced biopharma infrastructure, strong research and development investments, and established vaccine manufacturing hubs. Serum-free and specialty media are particularly popular, and with government support and fast innovation, they stay at the top of the market in the region.
For instance, in January 2026, Fujifilm has opened a new cell culture manufacturing plant in North Carolina, which will help it make more biopharmaceuticals. The site makes it easier to make vaccines and biologics, which helps meet the needs of healthcare around the world. Fujifilm's investment shows that they are committed to coming up with new ideas, using the latest manufacturing techniques, and meeting the growing need for reliable biopharmaceutical supply chains.
Asia Pacific is expected to exhibit the fastest growth, due to the growing biopharma infrastructure, the rising production of vaccines in India and China, and the strong investments made by the government. The area is the fastest-growing market in the world because healthcare needs are rising, people want things to be affordable, and new media technologies are being used quickly.
For example, Terumo unveiled with the Immucise™ Intradermal Injection System in July 2025. This system is meant to deliver approved drugs and vaccines directly into the skin. The device boosts the immune response, cuts the amount of vaccine needed by up to 80%, and saves money. The goal is to make global vaccination efforts safer, easier to get to, and more effective.
The U.S. will need a lot of cell culture media for vaccines in 2026 because it has a strong biopharma infrastructure, a lot of money going into research and development, and established vaccine manufacturing hubs. This is because of the rise of animal-free and specialty media, government support, and ongoing innovation. This ensures a steady supply for expanding immunization programs across the country.
For instance, in January 2026, Upside Foods is expanding beyond cultivated meat with the launch of Lucius Labs, a new company that makes cell culture media for life sciences. The goal of the move is to use its knowledge to help the biopharma and research industries, which will create new opportunities for sustainable innovation and advanced biotechnology applications around the world.
Australia's need for cell culture media in vaccines increases in 2026 when the country's first cell-based vaccine manufacturing facility opens in Melbourne. This milestone helps local production, makes us less dependent on imports, and helps us get ready for a pandemic. Strong growth in the country's biopharma sector is driven by government investment and new ideas in biotechnology.
For instance, in December 2025, Melbourne opened the first cell-based vaccine manufacturing facility in the Southern Hemisphere. This is a big step forward for Australia's biotech industry. The site makes it easier to make vaccines locally, lessens the need for imports, and helps keep the world healthy. This investment shows that Victoria is serious about innovation, advanced manufacturing, and being ready for the next pandemic.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 2,217.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 7.7% | 2033 Value Projection: | USD 3,727.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
HiMedia Laboratories, Thermo Fisher Scientific Inc., Merck KGaA, General Electric Company, Sartorius AG, Proliant, Inc., Bovogen Biologicals Pty. Ltd., Rocky Mountain Biologicals, Valley Biomedical, Moregate BioTech, Atlanta Biologicals, Creative-Biolabs, Life Technologies (India) Pvt Ltd., Axil Scientific Pte Ltd., Indian Immunologicals Limited, Serum Institute of India Pvt. Ltd., Sisco Research Laboratories Pvt. Ltd., and Valneva SE |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The rise in viral infections and new diseases has sped up the development of vaccines around the world. To stop pandemics and epidemics, governments and health organizations are putting considerable amounts of funds into immunization programs. This has led to a big rise in demand for reliable ways to make vaccines. Cell culture media are necessary for making vaccines, so this growing need is directly increasing the cell culture media for vaccine market share. This is because more pharmaceutical companies are using advanced cell-based technologies to meet the world's vaccination needs.
Growing percentage modern vaccines are made using cell-based methods instead of traditional egg-based systems. This makes them safer and higher efficiency. Cell culture media create controlled environments that make it easier to make vaccines with higher yields, more consistent results, and more room for growth. Continuous innovation in nutrient formulations, such as amino acids, vitamins, salts, and growth factors, improves production even more. These new developments are driving up the cell culture media for vaccines market demand. Biopharmaceutical companies are looking for specialized, high-performance media to support next-generation vaccines and meet the growing health needs around the world.
In the cell culture media for vaccine market forecast, investing funds into serum-free, chemically defined media is a key opportunity. Since these formulas do not include any animal-based ingredients, they are more consistent, less likely to get contaminated, and meet strict global regulatory standards. By using serum-free solutions, manufacturers can speed up the production of vaccines, make it easier to scale up, and meet the growing demand for safer biologics. This change not only makes the products better, but it also gives companies an edge in a market that is becoming more focused on following the rules and coming up with new ideas.
Specialized nutrient solutions that are necessary for growing cells used in vaccine research and production and manufacturing, is known as the cell culture media for the vaccine market. Most of the time, serum-free formulations are used because they are stable and less likely to get dirty. Serum-free media are a big part of the market because the industry likes defined compositions that make it easier to make vaccines that are compliant with regulations and can be made in large quantities. This change fits with the growing use of advanced cell culture systems, which use pure, controllable media environments to make cell lines work better in processes like viral replication and recombinant protein expression.
Market volumes show that the market is growing quickly every year. By 2024, the global demand for value scale will be close to one billion units. This is because greater resources is being put into biologics, mRNA, and viral vector vaccine platforms. North America is the region with the most demand, making up about four out of every ten units used around the world, driven by the biopharmaceutical infrastructure is well-developed and there is a significant amount of bioprocessing going on. Europe and Asia-Pacific together make up a large part of the remaining demand, reinforced by manufacturing capacity is increasing and governments are working to improve vaccine production capabilities.
There are only a few big manufacturers that make most of the media formulations, which shows that there are high technical barriers and specialized knowledge needed to make good media formulations. Some new things that are happening in innovation are custom media for certain cell lines and formats that work with continuous processing systems. These things make production yields better and give businesses more freedom to change the manner in which they do things.
Overall, the cell culture media for the vaccine market shows that more advanced formulations are being used, there are strong regional production hubs, and technology is always getting better, which makes the processes for making vaccines more reliable and efficient.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients