Global DNA repair drugs market is estimated to be valued at USD 8.05 Bn in 2025 and is expected to reach USD 19.29 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 13.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Global DNA repair drugs market is expected to witness robust growth over the forecast period due to factors such as increasing prevalence of cancer burden worldwide and rising demand for targeted therapies to repair defects in DNA damage response pathways in cancer cells. Furthermore, ongoing research activities in fields like CRISPR-based technologies for precise and customized DNA repair approaches can drive the market growth. However, lengthy drug development cycles and stringent regulations for these drugs can hamper the market growth. Partnerships between private and public entities for research funding and new product launches can drive the market growth.
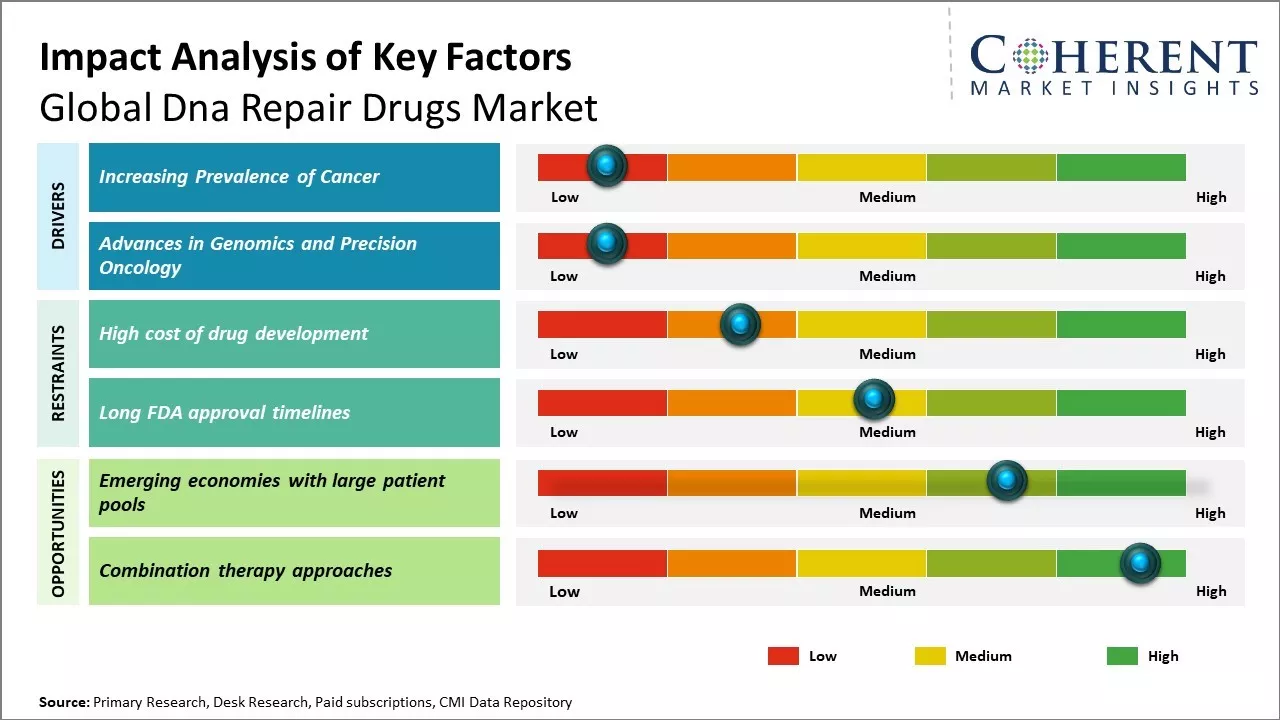
Increasing Prevalence of Cancer
Rising burden of cancer can drive the market growth. In 2020, an estimated 19.3 million new cancer cases and 10 million cancer deaths occurred worldwide. The five-year prevalence of cancer for the same year was estimated to be over 43.8 million people. If preventive measures are not taken or medical breakthroughs are not made, the global cancer burden will increase in the future due to rapidly aging populations and adoption of cancer-causing lifestyle choices such as smoking. Cancer develops due to damage caused to the DNA of healthy cells, either due to external factors like tobacco use, chemicals, radiation or due to internal factors like inherited genetic mutations, hormones, or immune conditions. When DNA damage occurs, it is vital that DNA repair mechanisms function properly in the cells to repair DNA and prevent uncontrolled cell growth. However, as cancer incidence rises worldwide, more and more people are developing DNA mutations that DNA repair mechanisms are unable to fix, thus, resulting in cancer. This has created a massive clinical need for drugs that can specifically target DNA repair pathways and help repair DNA damage, thus, preventing cancer onset or progression. Pharmaceutical companies are ramping up research efforts to develop innovative DNA repair drugs to address this unmet need. For instance, according to the data published by the ICMR-National Cancer Registry Programme, in December 2022, 1,461,427 cancer cases were reported in India, as compared to 1,426,447 cases in 2021. Rising cancer incidence rate boosts demand for DNA repair medications, and this can drive the market growth. Growing industry focus on the creation and research of innovative cancer treatment medications can also drive the market growth. In August 2021, Artios Pharma successfully raised EUR 129 million in a Series C fundraising round to develop cancer treatments that specifically target the cell's DNA repair machinery.
Get actionable strategies to beat competition: Download Free Sample
Advances in Genomics and Precision Oncology
The field of oncology is undergoing a revolution due to advances in genomics and precision medicine approaches. Next generation sequencing technologies enable comprehensive molecular profiling of tumor DNA and RNA to identify genetic alterations and mutations associated with specific cancer types. This provides deeper insights into tumor biology and elucidate the role of defective DNA repair mechanisms in cancer development. Large scale cancer genome profiling efforts have uncovered a large number of genetic mutations affecting DNA repair genes like BRCA1, BRCA2, ATM, and others across many different cancer types. By uncovering the specific DNA repair defects present in a patient’s tumor, precision oncology aims to match targeted therapies to these defects. Several DNA repair inhibitors are already being tested in clinical trials, particularly for cancers with homogeneous DNA repair defects like BRCA mutations in breast and ovarian cancer. As genomic profiling becomes more widespread, there will be increase in number of patients identified with DNA repair pathway alterations who may benefit from these therapies. Pharmaceutical companies are tremendously encouraged by the initial promising results with DNA repair drugs in trials.
Key Takeaways from Analyst
Global DNA repair drugs market growth is driven by rising incidence of cancer worldwide. Damaged and mutated DNA leads to unregulated cell growth and division, which can result in cancer if left unrepaired. Increasing focus on targeted therapies to treat various cancer types can offer opportunity for DNA repair drugs.
North America currently dominates the market due to growing R&D investments and rising acceptance of novel drug therapies. However, Asia Pacific is expected to emerge as the fastest growing regional market due to rapidly growing healthcare industry, rising healthcare spending, and increasing awareness about cancer treatment in countries like China and India.
High costs associated with drug development can hamper the market growth. Toxic side effects of certain drugs and efficacy/safety challenges related to newer therapies is a huge concern for patients. Furthermore, lengthy drug approval process also increases the development costs.
Increasing cancer burden worldwide and ongoing R&D to develop safer and more effective DNA repair drugs can drive the market growth over the forecast period. Collaborations between drug developers and healthcare providers can help address challenges and drive the market growth.
Market Challenges: High cost of drug development
The high cost of drug development can hamper the global DNA repair drugs market growth. Developing an effective drug from the stage of discovery to approval is an expensive process that requires extensive research, testing, and clinical trials. On average, it costs over US$ 2.6 billion to develop a new drug and take it through the entire clinical trial process for approval, according to a report published by the Tufts Centre for the Study of Drug Development in 2020. This massive financial investment combined with long development timelines deters many pharmaceutical companies from investing in DNA repair drugs. DNA repair is a complex mechanism involving many pathways and molecular targets. Drugs targeting DNA repair are often harder to develop as compared to other therapeutic areas as these must demonstrate efficacy in repairing genetic errors without causing unwanted side effects like increased cancer risk. This results in a high failure rate during clinical trials and contributes to the high costs. According to the data published by European Commission in 2021, nearly 75% of drug candidates fail during clinical trials despite showing promise in pre-clinical research, wasting millions of dollars in development costs. The financial risks involved discourage large pharmaceutical firms from aggressively investing in DNA repair therapeutics unless a very high return is expected.
Market Opportunities: Emerging economies with large patient pools
Emerging economies with their rapidly growing patient populations present a huge opportunity for the global DNA repair drugs market growth. Countries like India, China, Brazil and others in Asia Pacific and Latin America are experiencing significant economic development that improves access to healthcare for millions. As per the World Bank, over 300 million people in India and 200 million in China were raised out of extreme poverty between 2011-2019. This growing middle class now has more disposable income and healthcare remains an top priority. As life expectancies increase in these nations, common age-related diseases requiring DNA repair drugs like cancer also increases. Furthermore, governments in emerging economies have started prioritizing improvements to their public healthcare systems, which enhances diagnosis and treatment rates for various chronic diseases. For example, India’s National Health Policy 2017 aims to increase public healthcare spending to 2.5% of GDP by 2025 from the current 1% level. Programs like Ayushman Bharat are providing 500 million citizens access to health insurance coverage for the first time. Similarly, Brazil has had universal public healthcare since 1988. Its system SUS treats over 75% of the population. Improved access to diagnosis and care means more patients can be identified for DNA repair drugs if and when needed.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
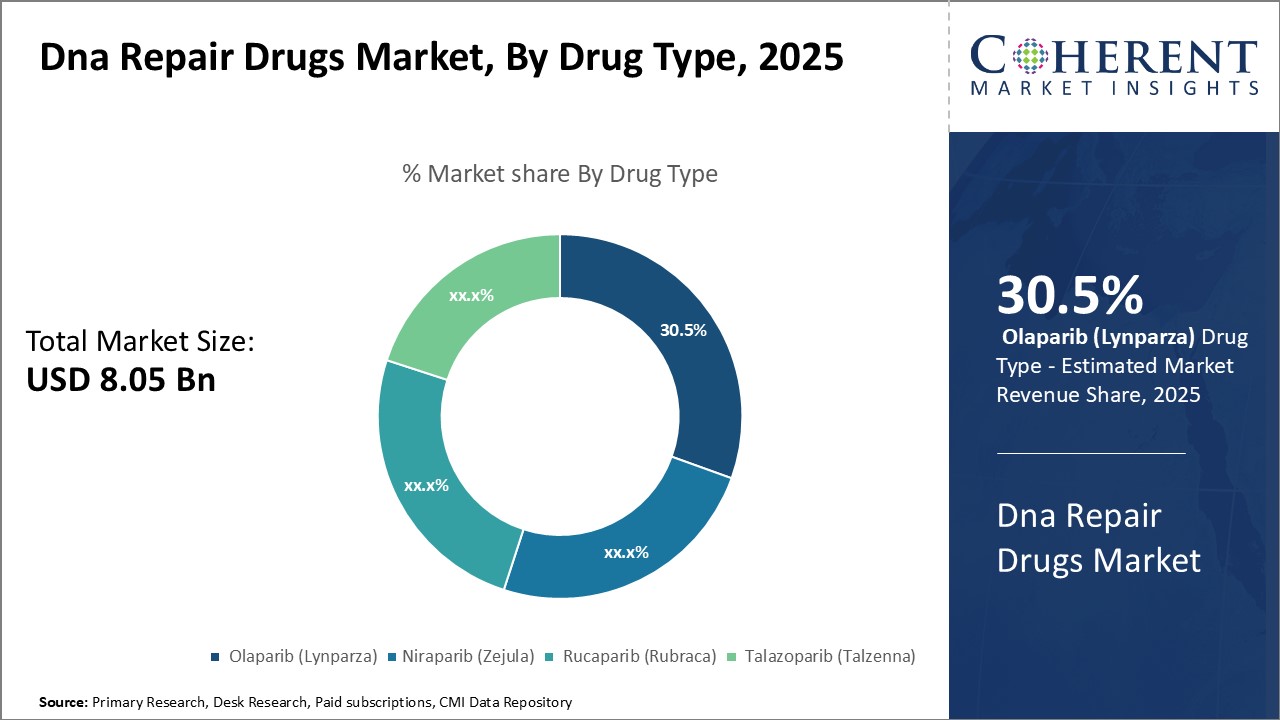
By Drug Type - Targeting resistant tumors:
In terms of drug type, Olaparib (Lynparza) segment is estimated to contribute the highest market share of 30.5% in 2025, owing to its ability to target tumors resistant to other therapies. Olaparib was the first PARP inhibitor approved for the treatment of cancer, receiving FDA approval in 2014 for recurrent ovarian cancer with germline BRCA1/2 mutations. It has also received additional approvals including approval in 2018 for HER2-negative metastatic breast cancer with germline BRCA1/2 mutations both as monotherapy and in combination with chemotherapy. This broad spectrum of approved indications has made it the leading PARP inhibitor in the market. Another advantage of Olaparib is its proven efficacy in treating homologous recombination deficient (HRD) tumors regardless of their BRCA mutation status, thus, significantly expanding the addressable patient population beyond just those with germline BRCA1/2 mutations. Furthermore, studies have shown Olaparib can inhibit tumor growth even in cancers that have developed resistance to other therapies such as platinum drugs or poly (ADP)-ribose polymerase inhibitors. This ability to target resistant tumors provides Olaparib an edge over other PARP inhibitors.
By Application - Dominating in breast cancer:
In terms of application, breast cancer segment is estimated to contribute the highest market share of 35 .21.5% in 2025, due to high prevalence of breast cancer globally as well as the role of DNA damage repair pathway defects such as BRCA1/2 mutations in a significant percentage of breast cancer cases. Drugs targeting DNA repair pathways like PARP inhibitors have emerged as an important treatment option for breast cancer patients with hereditary BRCA1/2 mutations. Olaparib has gained widespread adoption after receiving FDA approval for HER2-negative metastatic breast cancer with germline BRCA1/2 mutations. It has since become an established part of the standard of care for breast cancer patients with these mutations in both early and advanced settings. Olaparib's established efficacy and safety profile as well as the availability of a companion diagnostic test has positioned it as the preferred treatment option for addressing the unmet need in this large patient subset. This dominance of PARP inhibitors and Olaparib in BRCA-mutated breast cancer patients can drive the breast cancer segment growth.
By Route of Administration - Convenience of oral administration:
In terms of route of administration, oral administration segment is estimated to contribute the highest market share of 80.5% in 2025, as oral drugs are generally preferred over intravenous or parenteral options by both patients and physicians due to the convenience these offer. Patients find oral drugs less invasive and more discreet to take at home as compared to therapies requiring hospital visits for administration. Physicians also prefer prescribing oral therapies when possible due to lower risk of infusion reactions or catheter-related complications compared to parenteral drugs. Among the available DNA repair drugs, Olaparib was the first PARP inhibitor to receive approval in oral tablet form. This provided an important non-invasive treatment option for patients. The oral availability and administration of Olaparib avoids complications associated with intravenous routes while maintaining similar clinical efficacy. This has boosted demand for the drug through oral administration as compared to other routes. Olaparib's established status as an efficacious and convenient oral therapy has made it the standard of care for its approved cancer indications.
Need a Different Region or Segment? Download Free Sample
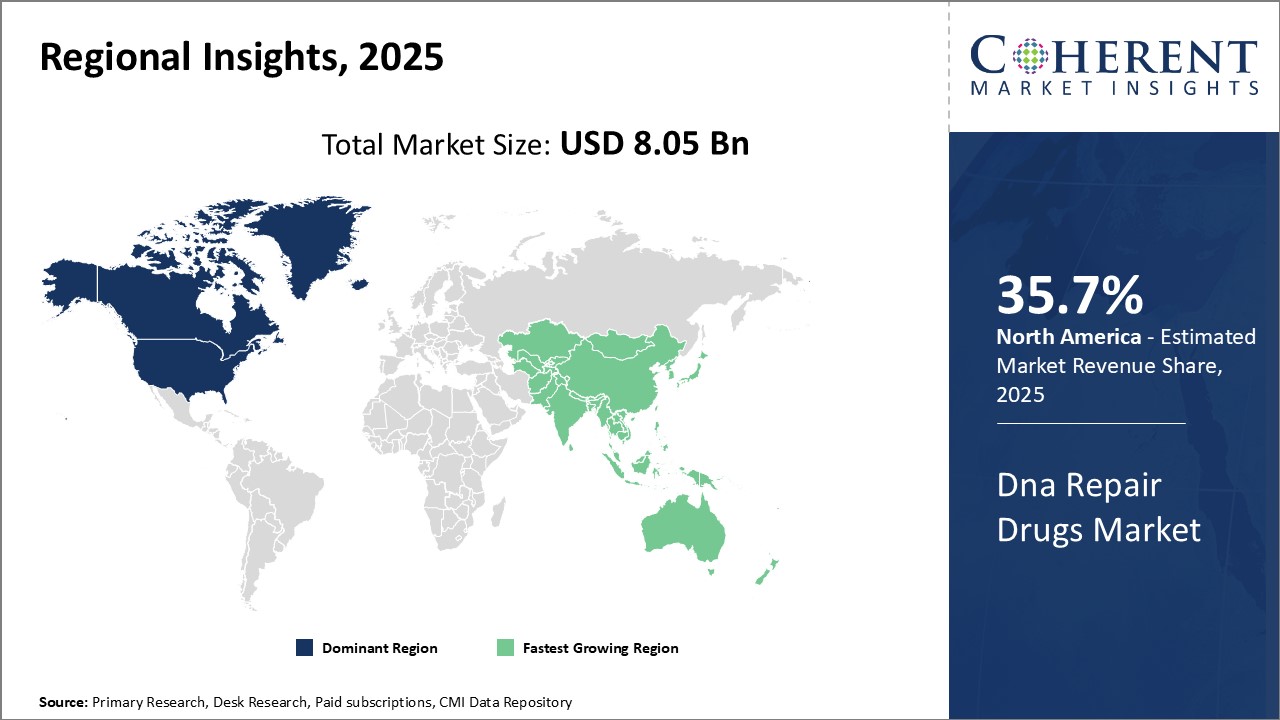
North America is estimated to contribute the highest market share of 35.7% in 2025. It dominate the global DNA repair drugs market. With the presence of major pharmaceutical companies and research institutions, the U.S. and Canada have emerged as global leaders in DNA repair drug development and manufacturing. Several companies in North America were among the earliest to recognize the potential of DNA repair pathways in treating cancer and developing targeted therapies. This region is also experiencing considerable funding for DNA repair research from both private and government sources, providing a fillip to ongoing clinical trials and new drug launches.
Asia Pacific represents the fastest growing regional market. Growing prevalence of cancer across Asian countries due to lifestyle changes, improved diagnostic rates and high unmet needs boosts demand for advanced treatment approaches. Local pharmaceutical players are increasingly partnering with global innovators to gain expertise in DNA damage response pathways. At the same time, countries like China and India offer a relatively favorable regulatory landscape to fast-track the clinical development of import DNA repair inhibitors. The lower costs associated with conducting late-stage trials have attracted several multinational sponsors to the Asia Pacific region in recent times. Furthermore, patents expiring in the near future can also facilitate the market entry of affordable biosimilar drugs in the DNA repair segment in Asia.
DNA Repair Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.05 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.3% | 2032 Value Projection: | USD 19.29 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
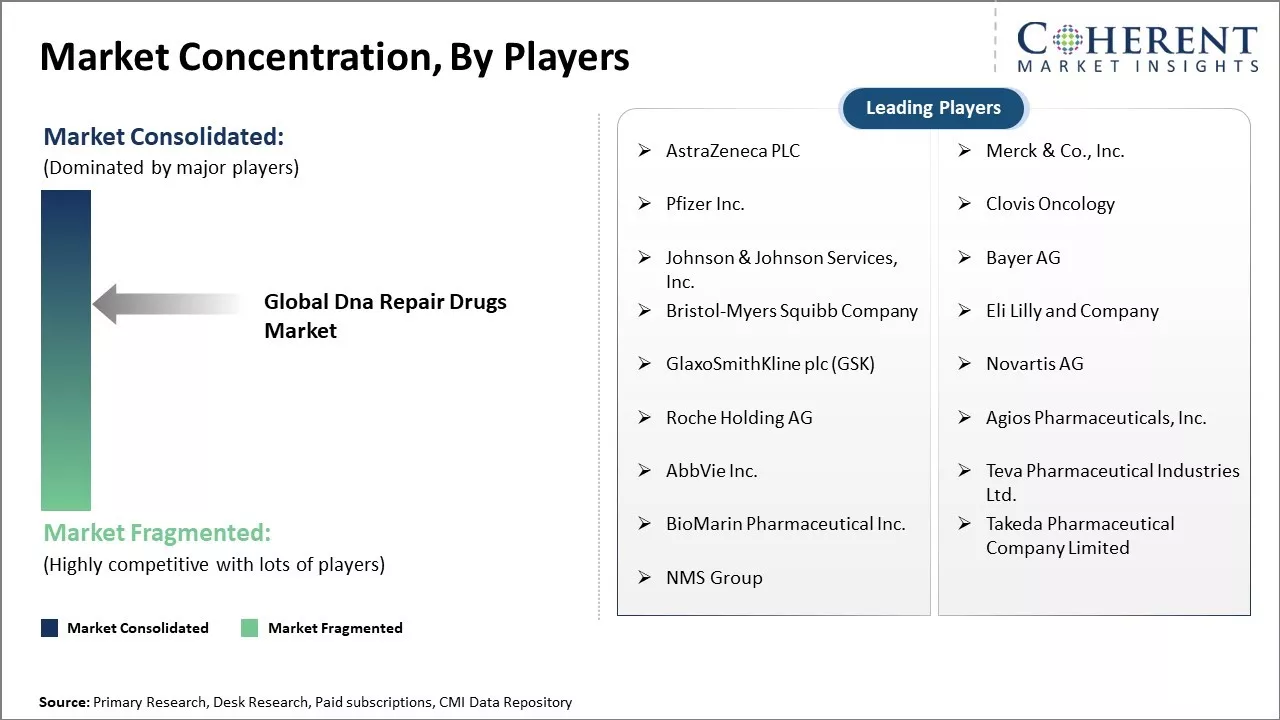
| Companies covered: |
AstraZeneca PLC, Merck & Co., Inc., Pfizer Inc., Clovis Oncology, Johnson & Johnson Services, Inc., Bayer AG, Bristol-Myers Squibb Company, Eli Lilly and Company, GlaxoSmithKline plc (GSK), Novartis AG, Roche Holding AG , Agios Pharmaceuticals, Inc., AbbVie Inc., Teva Pharmaceutical Industries Ltd., BioMarin Pharmaceutical Inc., Takeda Pharmaceutical Company Limited, NMS Group |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global DNA Repair Drugs Market involves pharmaceutical drugs that are used to repair damages in the DNA of cells. These drugs help treat diseases and conditions caused by defects in normal DNA repair mechanisms in the body. The DNA repair drugs market covers a range of prescription medication used for cancer treatment, genetic repair therapy, and other clinical applications that require DNA or gene damage repair. The global market covers development, manufacturing, and sale of DNA repair drugs around the world.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients