Genetic Chronic Obstructive Pulmonary Disease (COPD) Market is estimated to be valued at USD 615.5 Mn in 2025 and is expected to reach USD 767.3 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 3.2% from 2025 to 2032. Genes are the instructions or coding that human body's cells follow to give us characteristics like blue eyes, black hair, etc. We say that our skin tone or another feature is inherited or genetic since we receive half of our genes from each parent when we are born. Alpha-1 lung disease is frequently referred to as ‘genetic COPD’ because it is genetic. Alpha-1 lung disease patients have two faulty genes (one from each parent). The Z and S genes are the most prevalent aberrant genes. The initial diagnosis of alpha-1 frequently includes asthma or COPD brought on by smoking (COPD). Emphysema and chronic bronchitis are both aspects of COPD. The most frequent genetic risk factor for COPD is alpha-1. Alpha-1 may be present in up to 3% of individuals with COPD who have not been tested.
Figure 1. Global Genetic Chronic Obstructive Pulmonary Disease (COPD) Market Share (%), by Distribution Channel, 2025
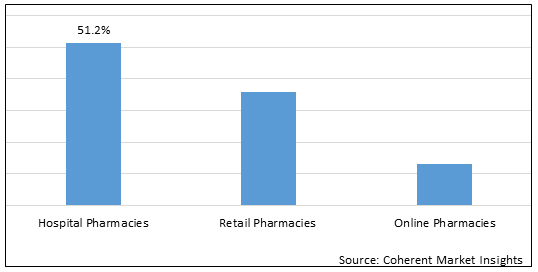
To learn more about this report, Download Free Sample
Genetic Chronic Obstructive Pulmonary Disease (COPD) Market: Drivers
Increasing prevalence of chronic obstructive pulmonary disease (COPD), associated with awareness efforts carried out by individual organizations is expected to drive the global genetic chronic obstructive pulmonary disease (COPD) market growth over the forecast period.
Increasing prevalence of chronic obstructive pulmonary disease is expected to drive the global genetic chronic obstructive pulmonary disease (COPD) market growth over the forecast period. For instance, according to fact sheet published by World Health Organization in May 2022, Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, causing 3.23 million deaths in 2019. Moreover, in November 2021, according to the data published by the National Heart, Lung and Blood Institute, each November, the chronic obstructive pulmonary disease (COPD) community comes together to promote better understanding of chronic obstructive pulmonary disease (COPD), a progressive lung disease that affects a lot of people across the U.S. Increasing awareness about chronic obstructive pulmonary disease (COPD) and its symptoms is important because early diagnosis and treatment can improve quality of life.
Figure 2.Global genetic chronic obstructive pulmonary disease (COPD) Market Share (%), by Region, 2025
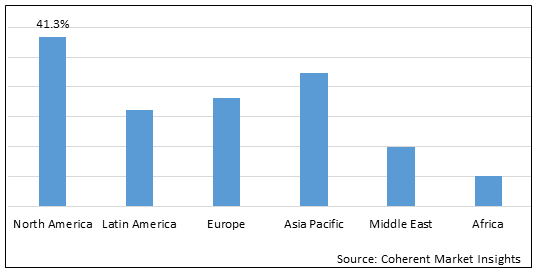
To learn more about this report, Download Free Sample
Increasing research and development activities and product launches for treatment of chronic obstructive pulmonary disease are expected to drive the global genetic chronic obstructive pulmonary disease (COPD) market growth.
Increasing research and development activities by key players in market for the development of awareness and product launches is expected to drive the global genetic chronic obstructive pulmonary disease market growth. For instance, in June 2022, Verona Pharma, a pharmaceutical company, announced completion of the patient enrolment, with more than 800 subjects involved for its randomized ENHANCE-1 trial. The study will evaluate ensifentrine for the maintenance treatment of chronic obstructive pulmonary disease. It is a critical step for the phase III ENHANCE trial with top-line data expected by the end of the year 2022 and further data from ENHANCE-2 in the third quarter of 2022.
Moreover, Verona Pharma, a pharmaceutical company, in May 2019, commenced a phase IIb clinical trial to assess nebulized ensifentrine (RPL554), in combination with a long-acting bronchodilator to treat moderate to severe chronic obstructive pulmonary disease.
Global genetic chronic obstructive pulmonary disease (COPD) Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
However, the COVID-19 pandemic had a positive impact on the global genetic chronic obstructive pulmonary disease (COPD) market, owing to the increasing demand of drugs used in the treatment of chronic obstructive pulmonary disease in patients infected with COVID-19 infection. For instance, according to an article published by the National Center for Biotechnology Information, providing access to biomedical and genomic information, in February 2022, chronic respiratory diseases (CRDs) affect the airways and other structures of the lungs, same dysfunction in lungs is observed in the patients infected with the coronavirus. The patients that are infected with coronavirus basically suffer from similar symptoms like breathing difficulty, insufficient supply of oxygen rich blood to lungs and overall body as in chronic obstructive pulmonary disease. Demand for drugs used in the treatment of chronic obstructive pulmonary disease increased as earlier in first wave of COVID-19 there was no particular drug therapy for treatment of COVID-19. The drugs used in treatment of chronic obstructive pulmonary disease were initially used in the treatment of coronavirus.
Genetic Chronic Obstructive Pulmonary Disease (COPD) Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 615.5 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 3.2% | 2032 Value Projection: | USD 767.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
GSK Plc, AstraZeneca Plc, Merck (Sigma-Aldrich), Dey Pharma, Grifols, S.A., Teva Pharmaceutical Industries Ltd, Baxter, Boehringer Ingelheim International GmbH, Kamada Ltd, Takeda Pharmaceutical Company Limited, LFB SA, Abeona Therapeutics, Alnylam Pharmaceuticals, Inc., Vertex Pharmaceuticals Incorporated, Kedrion S.p.A., and Arrowhead Pharmaceuticals, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Genetic Chronic Obstructive Pulmonary Disease (COPD) Market: Key Developments
In May 2022, Alembic Pharmaceuticals Ltd, a pharmaceutical company, received final approval from the U.S. health regulator for its generic version of Arformoterol Tartrate inhalation solution indicated for long-term treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease. The approval by the U.S. Food & Drug Administration (U.S. FDA) for the abbreviated new drug application (ANDA) for Arformoterol Tartrate inhalation solution is for strength of 15 mcg (base)/2 mL unit-dose vial.
Global Genetic Chronic Obstructive Pulmonary Disease (COPD) Market: Restraint
The major factors that hinder growth of the global genetic chronic obstructive pulmonary disease (COPD) market include that the development of new drugs is a challenging task and drug failures (termination) in clinical trials. For instance, asthma drug benralizumab failed to decrease annual COPD exacerbation rates for patients with moderate to very severe COPD, in May 2019. The Phase III, randomized, double-blind, placebo-controlled, parallel-group clinical trials GALATHEA and TERRANOVA evaluated the efficacy and safety of benralizumab for the prevention of exacerbations in patients with moderate to very severe COPD.
Key Players
Major players operating in the global genetic chronic obstructive pulmonary disease (COPD) market include GSK Plc, AstraZeneca Plc, Merck (Sigma-Aldrich), Dey Pharma, Grifols, S.A., Teva Pharmaceutical Industries Ltd, Baxter, Boehringer Ingelheim International GmbH, Kamada Ltd, Takeda Pharmaceutical Company Limited, LFB SA, Abeona Therapeutics, Alnylam Pharmaceuticals, Inc., Vertex Pharmaceuticals Incorporated, Kedrion S.p.A., and Arrowhead Pharmaceuticals, Inc.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients