Graft Versus Host Disease Market Size and Forecast – 2026 – 2033
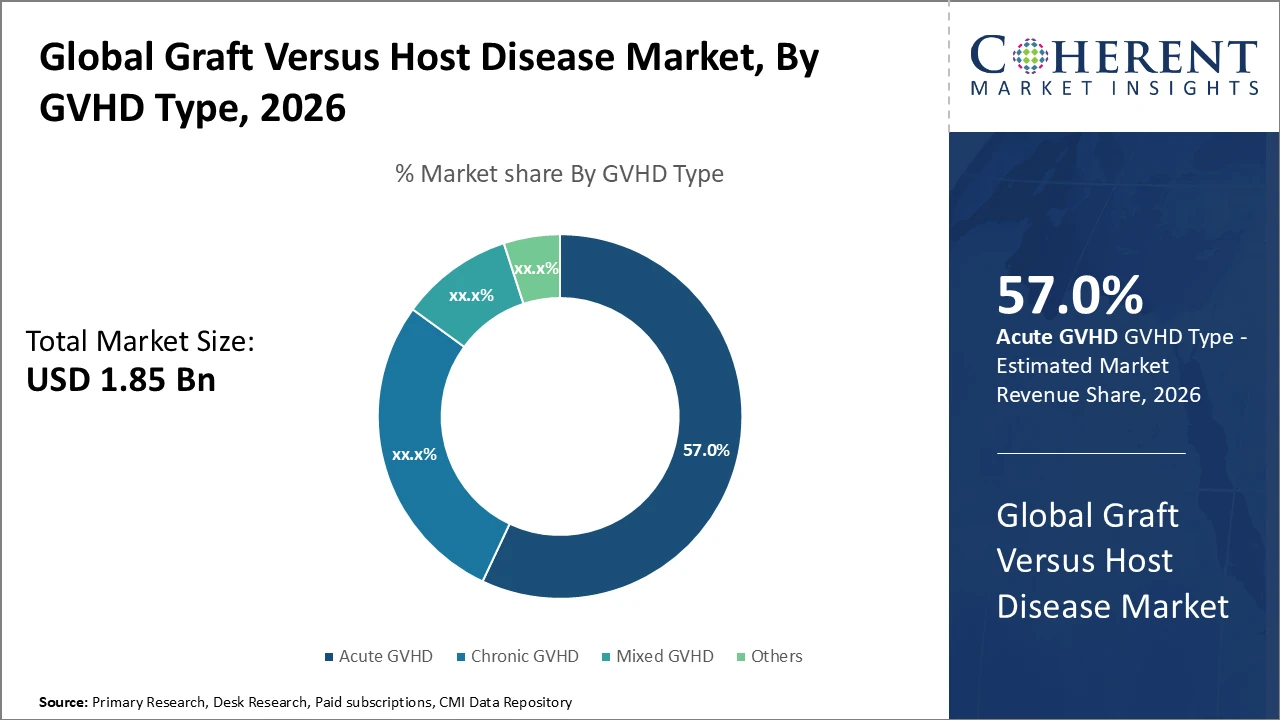
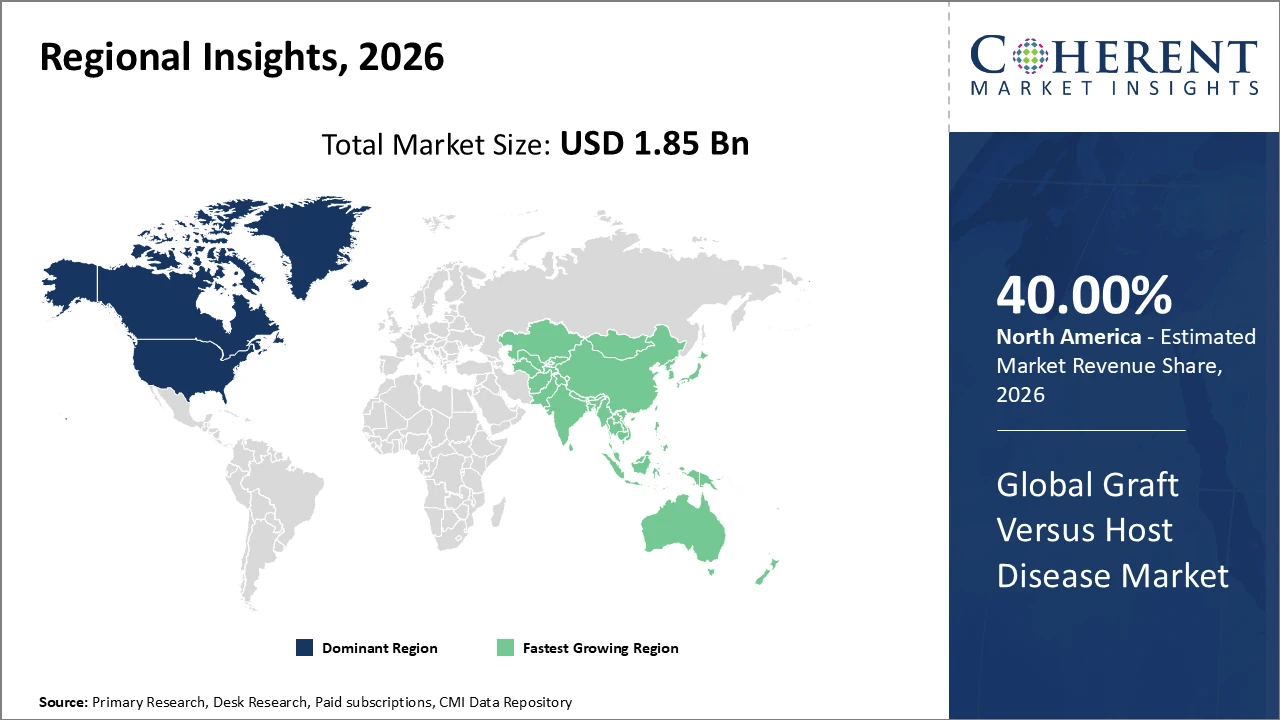
The global Graft Versus Host Disease market is estimated to be valued at USD 1.85 billion in 2026 and is expected to reach USD 3.65 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 10.2% from 2026 to 2033.
Global Graft Versus Host Disease Market Overview
The Graft Versus Host Disease (GVHD) market comprises a range of therapeutic products aimed at preventing or managing immune-mediated complications following allogeneic stem cell or bone marrow transplants. Key products include immunosuppressive agents such as corticosteroids, calcineurin inhibitors (e.g., cyclosporine, tacrolimus), and mTOR inhibitors, which help control donor T-cell activity. Monoclonal antibodies and biologics, including anti-thymocyte globulin and novel cytokine-targeting therapies, are increasingly used for steroid-refractory cases. Emerging treatments focus on cellular therapies, such as regulatory T-cell approaches, and small-molecule inhibitors that modulate immune signalling. These products collectively aim to reduce GVHD severity, improve patient outcomes, and expand the therapeutic pipeline.
Key Takeaways
The Acute GVHD segment leads in market revenue due to high incidence after allogeneic transplantation and ongoing innovation in targeted therapies improving survival rates.
Pharmacological therapies hold a dominant market share, supported by established clinical efficacy and an expanding pipeline of drugs enhancing treatment outcomes.
Hospitals and specialty clinics represent the largest end-user segment, benefiting from advanced infrastructure and availability of specialists for GVHD management.
North America dominates the Graft Versus Host Disease market, accounting for over 40% of the industry share, driven by advanced healthcare systems and extensive research initiatives.
Asia Pacific is the fastest-growing region, fueled by increasing transplantation volumes and improved access to novel therapies through supportive government policies, with a CAGR of nearly 13% from 2026 onwards.
regulatory frameworks that encourage the adoption of assistive technologies.
Graft Versus Host Disease Market Segmentation Analysis
To learn more about this report, Download Free Sample
Graft Versus Host Disease Market Insights, By GVHD Type
Acute GVHD dominates the market share due to its higher prevalence immediately after allogeneic transplantation and the urgent need for effective management. Clinical data indicate that acute cases make up around 58% of total GVHD occurrences, driving strong demand for corticosteroids and emerging immunosuppressive therapies. Chronic GVHD, although slower to develop, is among the fastest-growing subsegments as the number of long-term transplant survivors rises, creating demand for prolonged care. Mixed GVHD and other rare forms remain niche segments but are gaining attention with improved diagnostics. Chronic GVHD is increasingly managed with cellular therapies and novel biologics, reflecting tailored treatment approaches.
Graft Versus Host Disease Market Insights, By Treatment Type
Pharmacological therapy dominates the graft-versus-host disease (GVHD) treatment market, accounting for the largest share due to its established efficacy in managing both acute and chronic cases. This segment primarily includes immunosuppressants, corticosteroids, and targeted biologics, which effectively control donor immune responses and reduce tissue damage. Immunosuppressants such as cyclosporine and tacrolimus form the cornerstone of therapy, while corticosteroids provide rapid symptom relief. The strong clinical adoption of these drugs, supported by extensive research and treatment guidelines, reinforces pharmacological therapy as the preferred approach. Emerging biologics and combination regimens further enhance the effectiveness of this dominant treatment type.
Graft Versus Host Disease Market Insights, By End-User
Hospitals dominate the market share at approximately 45% due to high demand for institutional-grade devices aiding acute and chronic care patients. Rehabilitation Centers represent the fastest-growing subsegment, bolstered by increasing focus on post-injury recovery and the integration of novel therapeutic equipment using AI-driven devices. Home Care is expanding steadily as elderly and disabled individuals seek independent living supported by assistive technologies. Personal-use devices maintain a niche but growing presence through consumer-driven adoption of hearing aids and communication aids, reflecting rising market awareness and accessibility.
Graft Versus Host Disease Market Trends
Emerging trends in GVHD treatment show a shift toward precision medicine, using biomarkers to tailor immunosuppressive therapies to individual patient profiles.
Clinical trials from 2024 to 2026 indicate that biomarker-based treatments can reduce GVHD-related mortality by up to 25%.
Cellular therapies, including regulatory T-cell (Treg) approaches, are transforming treatment from broad immunosuppression to targeted immune modulation, showing promise in refractory chronic GVHD cases.
Adoption of biosimilars in emerging markets is accelerating growth by providing more cost-effective treatment options.
Asia Pacific and Latin America are key regions benefiting from biosimilar-driven accessibility and market expansion.
Graft Versus Host Disease Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Graft Versus Host Disease Market Analysis and Trends
North America dominates the Graft Versus Host Disease market due to advanced healthcare infrastructure, strong transplantation programs, and significant R&D investments. The U.S., as the largest national market, benefits from extensive clinical trial activity and favorable reimbursement policies supporting novel GVHD therapies. Major players, including Bristol Myers Squibb and Pfizer, have made substantial investments in the region, driving innovation and expanding their product pipelines. These factors collectively strengthen North America’s leadership, ensuring high adoption rates and sustained market growth in GVHD treatments.
Asia Pacific Graft Versus Host Disease Market Analysis and Trends
Asia Pacific is the fastest-growing Graft Versus Host Disease market, with a CAGR exceeding 13%. Growth is driven by expanding healthcare access, rising transplantation rates in countries such as China and India, and supportive regulatory frameworks. The increasing presence of multinational pharmaceutical companies alongside emerging local biotech firms is enhancing market penetration and facilitating strategic business expansion. These factors collectively position the region as a key growth hub, with rising adoption of novel GVHD therapies and improved patient access fueling sustained market development.
Graft Versus Host Disease Market Outlook for Key Countries
USA Graft Versus Host Disease Market Analysis and Trends
The USA continues to be the largest contributor to global Graft Versus Host Disease market revenue, driven by a high volume of hematopoietic stem cell transplants and early adoption of innovative therapies. In 2026, more than 25,000 transplants were performed, with JAK inhibitors and cellular therapies gaining significant clinical use and insurance coverage. Collaborative efforts among leading pharmaceutical companies, research institutes, and the FDA have accelerated drug approvals and real-world evidence generation. These initiatives have enhanced treatment accessibility, improved patient outcomes, and reinforced the country’s leadership position, significantly boosting overall market growth and sustaining its dominance in the global GVHD landscape.
Germany Graft Versus Host Disease Market Analysis and Trends
Germany’s Graft Versus Host Disease market is a key contributor to Europe’s growth, supported by advanced healthcare infrastructure, well-established hematopoietic stem cell transplant programs, and strong clinical research capabilities. The country benefits from robust collaborations between hospitals, research institutes, and pharmaceutical companies, enabling rapid adoption of novel GVHD therapies. Acute GVHD dominates treatment demand, while chronic GVHD management is expanding with cellular therapies and biologics. Regulatory support and reimbursement frameworks facilitate patient access to innovative treatments. Technological advancements, including biomarker-driven personalized therapies, are shaping market trends, positioning Germany as a strategic hub for GVHD treatment development and adoption in Europe.
Analyst Opinion
Advanced immunosuppressive therapies are driving market growth, with increased adoption of agents like JAK and BTK inhibitors expanding treatment options. In 2025, global prescriptions for JAK inhibitors in GVHD treatment rose by 35% year-over-year, reflecting broader clinical acceptance.
Expansion of allogeneic transplant procedures is boosting market revenue, particularly in North America and Europe. The U.S. alone performed over 25,000 allogeneic stem cell transplants in 2024, increasing demand for GVHD management.
Rising diagnostic innovations support early detection and management, with biomarker profiling enabling precise interventions. Trials in 2026 showed a 20% reduction in severe GVHD cases due to biomarker-guided therapy.
Pricing strategies and reimbursement policies influence market dynamics, as updated frameworks in developed regions improve access. The EU’s inclusion of new GVHD agents under specialty drug reimbursement in 2025 led to a 15% increase in patient treatment uptake.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.85 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 10.2% | 2033 Value Projection: | USD 3.65 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Novartis AG, Pfizer, Inc., Sanofi S.A., Incycle Corporation, Genentech, Inc., Celgene Corporation, Amgen Inc., Adaptive Biotechnologies, Fate Therapeutics, Inc., Astellas Pharma Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Graft Versus Host Disease Market Growth Factors
The growing number of hematopoietic stem cell transplants, driven by expanding clinical indications, is a key growth factor for the GVHD market. Healthcare registries report annual transplantation rates increased by over 12% between 2024 and 2026 in developed regions. The development of targeted therapies, such as JAK inhibitors, which showed a 40% improvement in corticosteroid-refractory GVHD response rates in 2025 trials, is further accelerating growth. Rising government investments in orphan disease drugs, supportive reimbursement policies, and increased clinician and patient awareness about GVHD and early intervention are boosting diagnosis rates and early-stage treatment adoption worldwide.
Graft Versus Host Disease Market Development
In February 2025, Sanofi Healthcare India announced that it had received marketing authorization in India for Rezurock (belumosudil tablets) for the treatment of chronic graft-versus-host disease (GVHD) in patients aged 12 years and older.
Key Players
Leading Companies of the Market
Novartis AG
Pfizer Inc.
Sanofi S.A.
Incycle Corporation
Genentech Inc.
Celgene Corporation
Fate Therapeutics, Inc.
Amgen Inc.
Astellas Pharma Inc.
Adaptive Biotechnologies
Leaders in the Graft Versus Host Disease market have focused on combination therapy development and patient-centric clinical trials to strengthen market share. In 2025, Bristol Myers Squibb announced a strategic collaboration with Kite Pharma targeting CAR-T cell therapies to improve chronic GVHD treatment outcomes, positioning the company as a key innovator. Similarly, Novartis launched a global expanded-access program in 2026, accelerating real-world data collection to enhance patient access and support regulatory approvals. These initiatives reflect the growing emphasis on innovation, collaboration, and evidence-based approaches in driving market growth and improving patient outcomes.
Graft Versus Host Disease Market Future Outlook
The Graft Versus Host Disease market is expected to grow steadily, driven by increasing hematopoietic stem cell transplant volumes and the rising prevalence of acute and chronic GVHD. Advances in targeted therapies, including JAK inhibitors, BTK inhibitors, and cellular therapies, are expanding treatment options and improving patient outcomes. Precision medicine approaches, biomarker-guided therapies, and combination treatment strategies are shaping personalized care. Market growth is further supported by favorable reimbursement policies, government investments in orphan disease drug development, and rising awareness among clinicians and patients. Emerging markets, particularly in Asia Pacific and Latin America, are poised for rapid adoption, creating significant growth opportunities globally.
Graft Versus Host Disease Market Historical Analysis
The Graft Versus Host Disease market has witnessed steady growth over the past decade, driven primarily by the increasing number of allogeneic hematopoietic stem cell transplants and the growing recognition of GVHD as a critical post-transplant complication. Early reliance on corticosteroids and conventional immunosuppressants established foundational treatment protocols, while the introduction of targeted therapies like JAK inhibitors and monoclonal antibodies in the late 2010s expanded therapeutic options.
Sources
Primary Research Interviews:
Hematologists and Oncologists
Transplant Physicians
Clinical Immunologits
Hospital Pharmacy and Specialty Clinic Managers
Biotech and Pharmaceutical R&D Professionals
Databases:
FDA and EMA Databases
IQVIA
WHO Health Statistics
Magazines:
Cancer Therapy Advisor
BioCentury
Pharmaceutical Executive
The Scientist
Immuno-Oncology News
Journals:
Journal of Immunotherapy
Blood
Clinical Cancer Research
Frontiers in Immunology
Bone Marrow Transplantation
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
STAT News
Associations:
American Society of Hematology (ASH)
European Society for Blood and Marrow Transplantation (EBMT)
International Bone Marrow Transplant Registry
American Society for Clinical Oncology (ASCO)
National Marrow Donor Program (NMDP)
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients