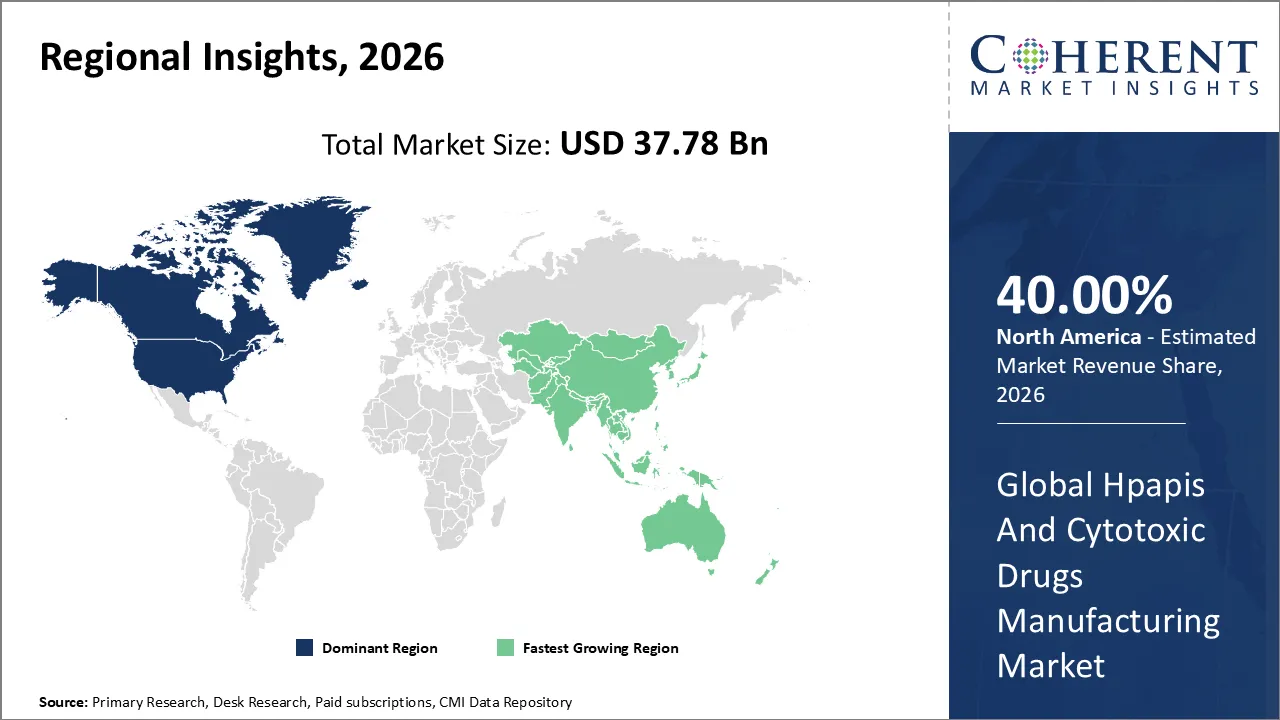
HPAPIS and cytotoxic drugs manufacturing market is estimated to be valued at USD 37.78 Bn in 2026 and is expected to reach USD 64.81 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 8.0% from 2026 to 2033.
Increase in demand for targeted therapy ingredients and oncology products is fueling the growth of the HPAPIs and cytotoxic drugs manufacturing market. There is a rise in the requirement for high-potency API ingredients used for cancer drugs, biologics, and antibody-drug conjugation. This increases the need for specialized facilities for HPAPIs. Furthermore, the pharmaceutical industry's research efforts, proprietary new drugs developed to treat different diseases, and regulatory support for new therapies are also contributing to the growth of this segment.
|
Current Events |
Description and its impact |
|
US-China Trade Relations and Regulatory Tensions
|
|
|
Global Cancer Drug Pipeline Expansion |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Based on drug origin, the chemical-based HPAPIs segment is estimated to hold the highest market share of 60% in 2026 due to existing well-established processes and sufficient market need in chemotherapy applications. Chemical-based HPAPIs find a wide range of applications in targeted therapy and chemotherapy formulations.
For instance, in January 2025, AGC Pharma Chemicals announced a major expansion in its HPAPI capabilities in Barcelona, Spain, to allow for seamless scale-up in the range from grams to tons in a single technology ecosystem.
Based on manufacturing location, the in-house manufacturing segment is projected to hold the highest market share of 55% in 2026. Major pharmaceutical companies interested in the in-house operation of high-potency API production due to the stringency in control, safety, and often proprietary formulations. The approach enables companies to improve compliance with regulatory guidelines on handling cytotoxic and ultra-potent APIs.
For instance, in February 2025, SK pharmteco unveiled a new CGMP‑compliant analytical testing laboratory at its Rancho Cordova, California site dedicated to High Potency Active Pharmaceutical Ingredients (HPAPIs).
By drug type, the novel HPAPIs segment is estimated to contribute the highest market share of 65% in 2026, driven by innovative therapies and patent-protected products. The need for next-generation oncology drugs and biologics rests on these novel HPAPIs, namely proprietary cytotoxic molecules and ADC payloads. High value and clinical importance create sustained demand, thus boosting the segment further.
For instance, in July 2025, Bristol‑Myers Squibb announced the launch of a new high potency API product to treat specific cancer types, further extending its commitment to the development of innovative oncology drug offerings and treatment of unmet medical needs in precision therapies.
Based on type of pharmacological molecule, the small molecules segment is projected to dominate with 80% market share in 2026, owing to their widespread use in cytotoxic and oncology drugs. Small molecules have continued to define high potency drug development, given their efficacy, relative ease of production, and application in targeted therapy.
For instance, in October 2025, Indena S.p.A. showcased improvements made to its GMP Synthetic plant near Milan, Italy, with a focus on the enlargement of its capabilities for the synthesis and production of small molecule HPAPIs, and complex and potent molecules, utilizing its improved technology for reactors and filtration dryers.
In terms of application, the oncology segment is anticipated to lead with 70% share in 2026, reflecting high demand for cancer-targeted therapies. HPAPIs and cytotoxic drugs are predominantly used in chemotherapy, ADCs, and other anti-cancer treatments, making oncology the most critical application segment in this market.
For instance, in April 2025, the FDA approved multiple oncology treatments, such as nivolumab (Opdivo) plus ipilimumab (Yervoy) for hepatocellular carcinoma and colorectal cancer.
To learn more about this report, Download Free Sample
North America is anticipated to maintain its leading position in the HPAPIs and cytotoxic drugs manufacturing market, accounting for 40% of the market share by 2026. The established base of pharmaceutical and biotechnology companies, advanced high-potency API manufacturing facilities, and high-end R&D capabilities in oncology and targeted therapies drive the leading position of the region.
For instance, in March 2025, Lonza Group expanded its high-potency API manufacturing facility in Portsmouth, New Hampshire, to enhance production capacity for oncology and cytotoxic drugs.
The Asia Pacific is likely to register the fastest market growth due to the rise in pharmaceutical outsourcing, demand for generics in HPAPI, government initiatives to attract investments in biotech, and CDMO capacity in Asia’s growing markets, such as China, India, and Korea.
For instance, in August 2025, WuXi AppTec inaugurated a new HPAPI production unit in Shanghai, China, designed to support both clinical and commercial manufacturing of cytotoxic and oncology APIs.
The U.S. HPAPIs and cytotoxic drugs market is leading globally, driven by the presence of established pharmaceutical giants, advanced manufacturing infrastructure, and a strong pipeline of oncology and targeted therapies. With decades of R&D expertise and state-of-the-art containment technologies, the U.S. continues to dominate high-potency API production.
For instance, in March 2025, Lonza Group expanded its high-potency API manufacturing facility in Portsmouth, New Hampshire. The expansion enhances production capacity for oncology and cytotoxic drugs with advanced process safety measures.
Japanese pharmaceutical firms have emphasized high-quality and precision manufacturing for cytotoxic and high-potency APIs to appeal to small niche oncology therapies and innovative ADCs. Japan's tough regulatory environment means that partners can be assured of the strictest safety and quality standards.
For instance, in September 2024, CMIC Pharma Science Co., Ltd. expanded its HPAPI production line in Shizuoka to support complex oncology molecules and cytotoxics for clinical trials in Japan. This is reflective of Japan's dedication to precision-based manufacturing in HP APIs.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 37.78 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.0% | 2033 Value Projection: | USD 64.81 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceutical Industries Ltd., Pfizer, Inc., Lonza Group, CordenPharma International, Evonik Industries AG, Flamma Group, Merck KGaA, CARBOGEN AMCIS, Catalent, Inc., Piramal Enterprises Ltd., AbbVie Inc., Fareva Group, Cerbios-Pharma SA, Novasep, Ajinomoto Bio-Pharma, PCI Pharma Services, Sterling Pharma Solutions, Heraeus Holding, Polpharma Biologics, Helsinn Healthcare SA, Seqens, Cambrex Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The growing incidence rates of cancer cases across the global population have been a key driver in the demand for high-potency APIs and cytotoxic drugs. APIs are required for the development of drugs such as chemotherapy agents, targeted small molecules, and antibody-drug conjugates. It has been witnessed that the growing focus of pharmaceutical companies on precision medicine and precision oncology drugs has boosted the demand for highly potent APIs requiring low dose amounts. In the same regard, the growing R&D investments and clinical pipeline for oncology drugs are boosting demand for specialized high-potency APIs manufacturing facilities.
The market is expected to witness strong growth opportunities through the expansion of specialized HPAPI manufacturing infrastructure and increased outsourcing to contract development and manufacturing organizations (CDMOs). Handling high-potency and cytotoxic compounds requires advanced containment technologies, regulatory expertise, and skilled workforce, which many pharmaceutical companies prefer to access through specialized partners.
The volume-driven manufacturing of HPAPIs and cytotoxic drugs has long been irrelevant; execution capability, regulatory maturity, and containment expertise determine the order of things. This market has reached a stage where only manufacturers with deep technical knowhow and proven operational discipline can remain competitive. The shift toward targeted oncology therapies, antibody-drug conjugates, and precision small molecules has fundamentally changed expectations from API manufacturers-basic synthesis competence no longer suffices.
Another feature associated with this market is the process intricacy of novel HPAPIs, particularly cytotoxics, which find application in cancer therapy. In several instances, HPAPIs are associated with difficult reaction conditions, including unstable reactants, narrow reaction windows, as well as process scales that make use of multi-step synthetic pathways.
Regionally, established markets retain influence due to regulatory trust and IP protection, while emerging regions are progressing rapidly by aligning facilities with global compliance standards. However, in my assessment, capability parity does not automatically translate into sponsor confidence. Regulatory inspection history, transparency, and crisis management experience still heavily influence sourcing decisions for HPAPIs.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients