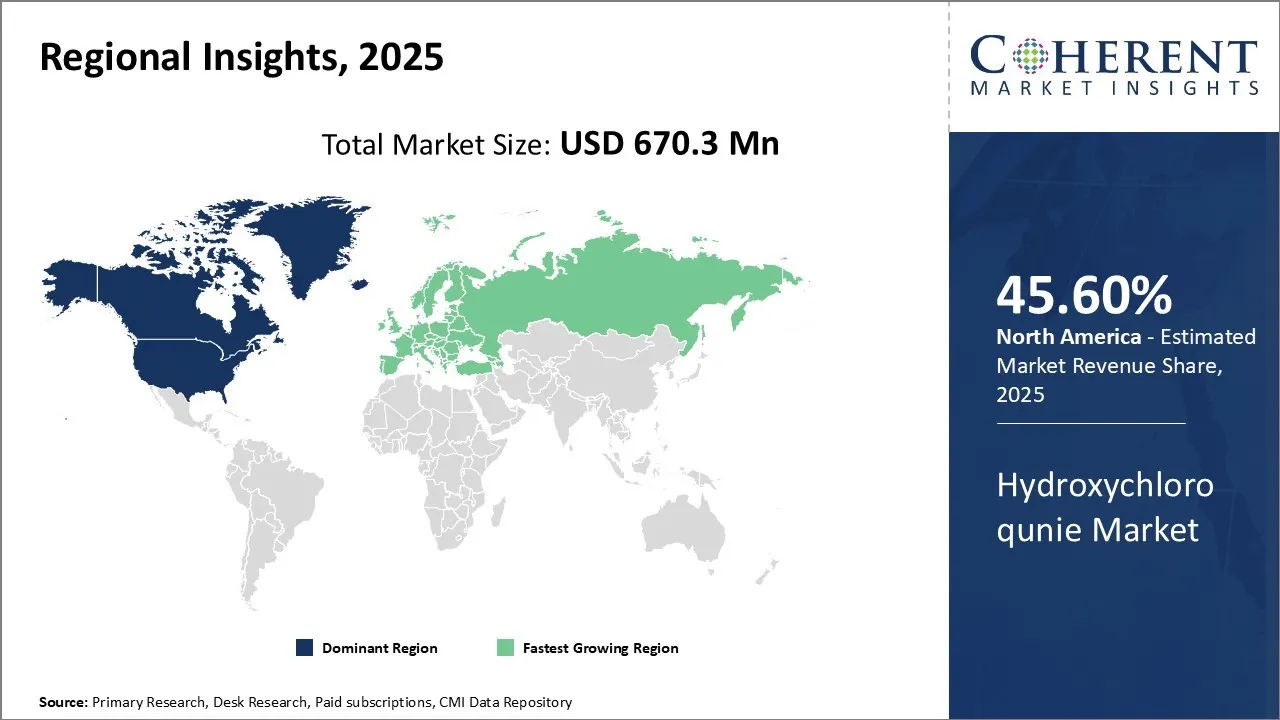
Hydroxychloroquine Market is estimated to be valued at USD 670.3 Mn in 2025 and is expected to reach USD 988.1 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.7% from 2025 to 2032.
The global hydroxychloroquine market is witnessing steady growth, it is mainly being fuelled by its extensive application in the management of autoimmune diseases, including rheumatoid arthritis, lupus erythematosus, and certain inflammatory diseases. Demand is stable due to the drug's well-established application in long-term chronic disease management regimens, particularly in populations with increasing age where autoimmune diseases are more common.
As of 2025, major producers like Sanofi and Zydus Cadila persisted in building market presence by securing regulatory approvals, expanding capacity, and R&D on biosimilar or modified-release products. These actions demonstrate a dedication to therapeutic innovation as well as market adaptability in the face of shifting global health dynamics.
|
Current Events |
Description and its impact |
|
Expanded Use of Hydroxychloroquine in Autoimmune Therapy Trials |
|
|
India's API Export Expansion for Hydroxychloroquine |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The worldwide pipeline for hydroxychloroquine market is still quite mature with minimal new drug development but more emphasis on formulation innovation and broadened therapeutic use. Pipeline activity currently focuses on the improved safety profile and treatment scope of hydroxychloroquine.
Some pharmaceutical firms like Sanofi, Novartis, and Zydus Lifesciences are investing in extended-release (ER) and targeted delivery systems to minimize adverse effects like retinal toxicity and cardiomyopathy. These new formulations seek to enhance compliance for long-term autoimmune diseases such as lupus, rheumatoid arthritis, and Sjögren's syndrome.
Clinical investigation also investigates hydroxychloroquine as a supportive treatment in cancer and viral disease, although its uses in these areas are still in initial stages and will need to overcome regulatory obstacles. Additionally, universities and CROs are investigating biomarker-based approaches to stratify patient subgroups for optimal benefit from hydroxychloroquine treatment, especially in precision medicine settings.
With generic players controlling the market, branded pipeline activity is weak; however, the attention has turned to differentiated generics and biosimilar approaches in highly regulated markets such as the U.S. and EU. Overall, although innovation is incremental, pipeline activity reflects a value extension strategy, a strategy of minimizing risk, and becoming aligned with changing global regulatory standards.
The international hydroxychloroquine market is largely generic-led, with original patents on hydroxychloroquine having run out decades earlier, and hence the molecule being off-patent and readily available. The competitive scene is therefore led by generic players like Zydus Lifesciences, Teva Pharmaceuticals, Mylan, and Sanofi, the original developer.
Recent patenting in this field is focused not on the active pharmaceutical ingredient (API) per se, but on formulation patents, process enhancements, and combination therapy. Companies are filing for intellectual property protection of changed-release forms, means of minimizing side effects, and co-administration regimens of hydroxychloroquine and other immunomodulatory agents.
For instance, patent filings in Europe and the U.S. demonstrate interest in minimizing retinal and cardiac toxicity by means of new delivery systems or adjuncts. The availability of products such as hydroxychloroquine's generic counterparts makes them more accessible worldwide.
Off-label and new therapeutic uses are also receiving increased interest in method-of-use patents, such as in certain viral diseases, cancers, and orphan autoimmune diseases. These, however, are under close scrutiny by the regulatory bodies and are subject to challenge because of the established reputation of hydroxychloroquine. The patent space is crowded at the molecular level but open at the formulation and use-based patent levels, where firms attempt to establish market exclusivity and clinical differentiation within a commoditized marketplace otherwise.
United States
Regulatory Agencies & Codes
- FDA Approval: Hydroxychloroquine (Plaquenil®) is approved by the FDA for use in lupus and rheumatoid arthritis but not COVID-19.
- NDC Codes:
- Hydroxychloroquine sulfate 200 mg tablet: 00004-0240-34 (brand), 00904-2402-61 (generic)
- CMS Guidelines: Medicare Part B/D covers the drug when used for FDA-approved indications, which requires prior authorization for off-label use.
Insurance Coverage & Spending
- Medicaid: Hydroxychloroquine for autoimmune diseases covered, with annual per-patient spending of ~$1,200 (80–90% coverage)
- Private Insurers: Non-lupus/rheumatoid arthritis prior authorization requirements. Blue Cross Blue Shield estimated $28M in 2022 hydroxychloroquine claims, 92% autoimmune diseases.
State-Level Restrictions
- New Hampshire: 30-day limits on new prescriptions (lupus patients exempt)
- New Jersey: Prevents prescriptions off FDA-approved use or clinical trials.
Asia
India
Regulatory Framework
- National List of Essential Medicines (NLEM): Hydroxychloroquine has been included for malaria and autoimmune conditions.
- Insurance: Public insurers (e.g., Ayushman Bharat) reimburse 50–70% of costs when approved. Private insurance normally excludes off-label prescriptions.
Japan
Reimbursement Process
- NHI Coverage: Authorized for lupus, rheumatoid arthritis, and malaria under NHI codes 6249101 (200 mg tablet).
- Cost Sharing: Patients contribute 30% (10% for chronic conditions under certain programs).
Africa
Challenges & Coverage
- Public Health Programs: Restricted reimbursement except for malaria prophylaxis (e.g., Nigeria's National Malaria Elimination Program).
- Private Insurance: Covers ~20–40% of costs, mainly in urban regions. Out-of-pocket expenses rule (80% of total)
Southeast Asia
Thailand
- Universal Coverage Scheme: Covers hydroxychloroquine for lupus and malaria (30 THB/$0.85 co-payment per prescription).
- Spending Data: Government spent ฿120M ($3.4M) on autoimmune indications in 2023.
Indonesia
- BPJS Kesehatan: Pays 60% of hospitalized lupus patient costs. Excludes outpatient prescription coverage.
Key Trends & Data Gaps
A more detail comparison of reimbursement policies is given below:
| Region | Coverage Scope | Agencies Involved | Avg. Patient Spending |
North America | Lupus, rheumatoid arthritis | FDA, CMS, State Medicaid | $1,200–$1,500/year [5] |
| India | Malaria, autoimmune diseases | NLEM, Ayushman Bharat | $200–$400/year |
| Thailand | Lupus, malaria | NHSO | $30–$50/year
Rheumatoid Arthritis (RA)
- Early RA: HCQ is usually initiated as an initial disease-modifying antirheumatic drug (DMARD) at 200–400 mg/day (usually 5 mg/kg real weight) Sample regimens:
- Monotherapy: Plaquenil (200 mg twice daily)
- Combination therapy: HCQ with methotrexate or sulfasalazine to augment effectiveness
- Moderate-to-severe RA: HCQ is used adjunctively with biologics (e.g., TNF-α inhibitors like adalimumab) or targeted synthetic DMARDs (e.g., JAK inhibitors)
Systemic Lupus Erythematosus (SLE)
- Mild SLE: HCQ monotherapy (200–400 mg/day) is standard for managing cutaneous and musculoskeletal symptoms
- Severe SLE (e.g., nephritis, CNS involvement): HCQ is combined with:
- Immunosuppressants: Mycophenolate mofetil or cyclophosphamide.
- Corticosteroids: Prednisone (short-term use to minimize toxicity)
Malaria
- Prophylaxis: Restricted to areas where there is no chloroquine resistance (400 mg weekly)
- Acute treatment: Reserved for uncomplicated P. vivax or P. oval infections (800 mg as a loading dose, followed by 400 mg at 6h, 24h, and 48h). Needs adjunctive primaquine for hypnozoite clearance.
Medications by Stage and Line of Treatment
| Condition | Stage/Line | Medications/Brands | Dosage |
| Rheumatoid Arthritis | First-line | Plaquenil (HCQ) ± methotrexate | 200–400 mg/day
| SLE | Maintenance | HCQ + belimumab (Benlysta) | 400 mg/day
| Malaria | Prophylaxis | Generic hydroxychloroquine | 400 mg/week
Key Factors Affecting Prescriber Preference
Safety and Monitoring
- Risk of retinopathy: Dosage limited to 5.0 mg/kg actual weight to limit cumulative toxicity. Periodic ophthalmologic examination is required.
- Interactions: HCQ effectiveness can be reduced with concomitant use of antacids or CYP450 inducers.
Regulatory and Access Factors
- Limits on prescription: Historically, some states limited HCQ dispensing to 14-day supplies in the setting of pandemics, but these policies have been largely repealed.
- Cost and insurance: Generic HCQ (avg $20/month) is used instead of brand-name Plaquenil ($150/month) where cost is an issue.
Therapeutic Timing
- Delayed onset: It takes HCQ 1–2 months to reach full effect, so concurrent use of corticosteroids or NSAIDs is needed for quick symptom control
Resistance Patterns
- Geographic chloroquine resistance in malaria restricts the use of HCQ to particular geographies (e.g., Central America)
Emerging Trends and Challenges
- Biologic combinations: Increased practice of HCQ with belimumab in SLE to decrease steroid reliance.
- Telemedicine monitoring: More remote retinopathy screenings to improve long-term compliance.
- Generic substitution: 90% of U.S. prescriptions are for generic HCQ because of payer mandates.
The growing prevalence of diseases like Psoriatic Arthritis across the world is expected to aid in growth of the global hydroxychloroquine market. For instance, according to an article published in Plos One- a open access journal, Spain had the highest cases of Psoriatic Arthritis in Europe, followed by Norway. The use of hydroxychloroquine, as a drug treatment for Psoriatic Arthritis can drive the growth of the global hydroxychloroquine market.
The increasing partnerships among pharmaceutical can drive the growth of the global hydroxychloroquine market. For instance, in July 2021, Sanofi- a France based multinational pharmaceutical and healthcare company and Asahi Kasei Corporation- a Japan based multinational chemical company, executed a marketing license agreement to transfer sales right in Japan for hydroxychloroquinine tablets. Sanofi remained as the marketing authorization holder of hydroxychloroquine Japan and continued manufacturing the product.
By Disease Indication, the malaria segment is expected to hold a dominant position in the global hydroxychloroquine market during the forecast period and this is attributed to high transmission rate of the disease.
By Distribution Channel, the hospital segment is expected to hold a dominant position in the global hydroxychloroquine market during the forecast period and this is attributed to increased hospitalizations.
Among disease indication segment, Rheumatoid Arthritis segment is estimated to dominate in North America over forecast period owing to the rowing prevalence of the disease. According to an article published in June 2020, by Medical News Today- web-based outlet for medical information and news, targeted at both the general public and physicians, 1.3 million people over the age of 65 are infected with Rheumatoid Arthritis every year
To learn more about this report, Download Free Sample
Among regions, North America is estimated to hold a dominant position in the global hydroxychloroquine market over the forecast period. This is due to the increasing number of research and development activities on diseases like Lupus Erythematosis in the U.S.
For instance, according to an article published on May 4, 2025, by Labiotech-a digital media platform focused at Biotech industry in Europe, the research by Vanderbilt University Medical Center- medical provider with multiple hospitals in the U.S, uncovered that patients with lupus have higher levels of iron in their T cells. This condition is called Hemochromatosis and can lead to general fatigue and joint pain. The use of hydrochloroquine to treat lupus can help balance iron levels among patients.
India is the world's largest producer and exporter of hydroxychloroquine, largely because of its well-established generic pharma industry and low-cost production capabilities. Large players such as Zydus Lifesciences, Ipca Laboratories, and Cipla account for most of API and finished formulation production. India's exports of hydroxychloroquine increased during the pandemic, solidifying its position as a major supplier to Asia, Africa, and Latin America.
The United States is the largest consumer of hydroxychloroquine, fuelled by extensive use in treating autoimmune diseases such as lupus and rheumatoid arthritis. Although domestic supply is available, the U.S. is largely reliant on imports, particularly from India. Authorities like the FDA have had a major role to play in regulating its use, especially following the controversial off-label prescribing during the COVID-19 pandemic.
China is also a major player in the hydroxychloroquine supply chain, especially upstream in producing raw materials and chemical intermediates for API manufacturing. Although China's contribution to finished formulation is minimal compared to India, China is crucial for upstream activities and thus a major partner or competitor in international pharmaceutical supply chains.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 670.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.7% | 2032 Value Projection: | USD 988.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Sanofi, Amneal Pharmaceuticals, LLC, Laurus Labs, Zydus Group, Prasco, LLC, Dr. Reddy's Laboratories, Ltd, Cadila Pharmaceuticals, Novartis AG, Concordia Pharmaceuticals Inc. (A subsidiary of Advanz Pharma), Covis Pharma GmbH, Cardinal Health, Aphena Pharma Solutions, Mylan N.V (A subsidiary of Vitris), McKesson Corporation, Teva Pharmaceutical Industries Ltd, Lupin, Sun Pharmaceutical Industries Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Major players operating in the global hydroxychloroquine market include Sanofi, Amneal Pharmaceuticals, LLC, Laurus Labs, Zydus Group, Prasco, LLC, Dr. Reddy's Laboratories, Ltd, Cadila Pharmaceuticals, Novartis AG, Concordia Pharmaceuticals Inc. (A subsidiary of Advanz Pharma), Covis Pharma GmbH, Cardinal Health, Aphena Pharma Solutions, Mylan N.V (A subsidiary of Vitirs), McKesson Corporation, Teva Pharmaceutical Industries Ltd, Lupin, Sun Pharmaceutical Industries Ltd.
Definition: Hydroxychloroquine is in a class of drugs called antimalarials and is also an antirheumatic drug. It works by killing the organisms that cause malaria. Hydroxychloroquine may work to treat rheumatoid arthritis and systemic lupus erythematosus by decreasing the activity of the immune system.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients