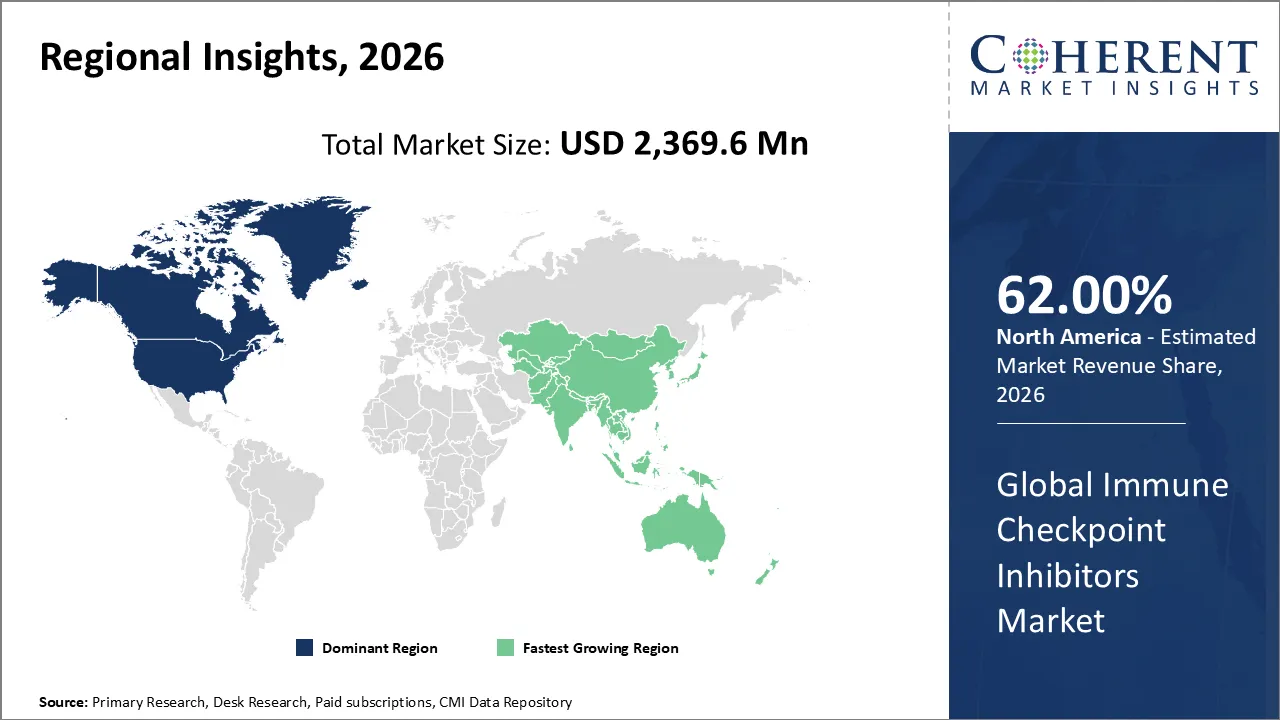
The global immune checkpoint inhibitors market was valued at USD 2,369.6 Mn in 2026 and is forecast to reach a value of USD 5,615.6 Mn by 2033 at a CAGR of 13.2% between 2026 and 203.
Immune checkpoint inhibitor products come under biologic therapeutic products. Biologic manufacturers require similar kind of approvals such as drugs from the U.S. Food & Drug Administration prior to bringing the product into market. However, unlike drugs, biologics require Biologics License Applications (BLA) to be filed with the Center for Biologics Evaluation and Research (CBER).
|
Current Event |
Description and its Impact |
|
US-China Trade Relations and Technology Transfer Restrictions |
|
|
Current Events and their Impacts |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
|
Category |
Data/Statistics |
|
Total Active Trials Worldwide |
~5,000+ clinical trials investigating ICIs |
|
Focus on PD‑1/PD‑L1 Inhibitors |
~60% of all ICI trials target PD‑1/PD‑L1 pathways |
|
Combination Therapies |
~70% of ongoing studies involve ICIs combined with chemotherapy, targeted therapy, or novel agents (e.g., IL‑15 superagonists) |
|
Next‑Gen Checkpoints |
Trials increasingly explore LAG‑3, TIGIT, and TIM‑3 inhibitors, expected to represent ~25% of the market by 2030 |
|
Cancer Types Studied |
NSCLC, melanoma, colorectal, bladder, and breast cancers dominate trial focus, accounting for ~75% of studies |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of distribution channel, the hospital pharmacies segment is projected to account for 72.4% share in 2026. Immune checkpoint inhibitors are given through an IV, requiring special oncology centers for safe delivery. Hospitals are still the main place for cancer treatment, which makes it easier to monitor on patients and make sure they follow through. This clinical dependency makes hospital pharmacies the most important players in the market, ahead of retail and online stores.
For instance, in January 2026, Sun Pharma's Cosibelimab (Unloxcyt) is a PD-1 immune checkpoint inhibitor that is now available in the U.S. for advanced cutaneous squamous cell carcinoma (CSCC). This launch gives patients with hard-to-treat skin cancers more treatment options. It also shows how PD-1 inhibitors are becoming of greater value in oncology and hospital-based cancer care.
In terms of drug class, the Programmed Death Receptor-1 (PD-1) Inhibitors segment is expected to hold 43.2% share of the market in 2026, because they are approved for use in many types of cancer. Keytruda and Opdivo are two flagship drugs that drive adoption since they have been shown to help people live longer. Their ability to work well in both monotherapy and combination regimens will keep them ahead of PD-L1 and CTLA-4 competitors.
For instance, in February 2026, the 2026 ASCO GU Symposium update from Merck is all about Keytruda (pembrolizumab), a PD-1 inhibitor that works on bladder and kidney cancers. The data support pembrolizumab's role in improving survival and response rates, often when used with drugs like Padcev and Welireg. This shows that PD-1 inhibitors are still the most important type of immune checkpoint therapy.
In terms of cancer type, the lung cancer segment is expected to lead the market with 37.6% share in 2026. There is global burden of non-small cell lung cancer in the world, and there is strong clinical evidence for PD-1/PD-L1 inhibitors, which drives demand. Lung cancer is more common than melanoma, renal, and other cancers as it is used in first-line treatment and expanding approvals.
For instance, in January 2026, the University of Miami article addresses Durvalumab (Imfinzi), a PD-L1 immune checkpoint inhibitor, as an important milestone forward in the treatment of small cell lung cancer. According to the ADRIATIC trial, using this drug as maintenance therapy after chemoradiation can greatly improve survival. This makes Durvalumab a promising new standard for treating lung cancer.
To learn more about this report, Download Free Sample
North America is expected to dominate the immune checkpoint inhibitors market with 62% share in 2026. High rates of cancer, a strong healthcare system, good reimbursement policies, and the presence of big pharmaceutical companies all contribute to this leadership. The U.S. has the largest portion because it has numerous instances of clinical trials and quick approvals from the government.
For instance, in February 2026, Exelixis declared that the U.S. FDA approved its new drug application for zanzalintinib in combination with an immune checkpoint inhibitor to treat metastatic colorectal cancer. This milestone shows the importance of checkpoint inhibitors in combination treatments that aim to help people with advanced, and hard-to-treat cancers.
Asia Pacific is expected to exhibit the fastest growth. In countries like China, Japan, and India, ICIs are being used progressively faster because of rising cancer rates, rising healthcare costs, better access to advanced therapies, and government efforts to improve cancer care. This makes Asia-Pacific the fastest-growing part of the industry.
For instance, in February 2026, Lotus Pharmaceutical and Formycon have teamed up to commercialize FYB206, a biosimilar candidate to Keytruda (pembrolizumab), in major Asia-Pacific markets. This partnership makes it easier for more people to get new cancer immunotherapies, which strengthens the role of PD-1 inhibitors in cancer treatment plans around the world. Keytruda is a leading PD-1 immune checkpoint inhibitor.
In 2026, the immune checkpoint inhibitors market is extremely prevalent in the U.S. because cancer is becoming more common, the healthcare system is strong, and the FDA approves drugs quickly. Strong clinical trial activity, good reimbursement policies, and the use of combination therapies all make it easier for patients to get the care they need, which leads to steady demand and market growth across the country.
For instance, in February 2026, ImmunityBio stated that the combination of Anktiva (nogapendekin alfa inbakicept) and immune checkpoint inhibitors worked well in U.S. trials for non-small cell lung cancer (NSCLC). Conducted in American patients, the studies showed enhanced immune restoration and survival benefits, underscoring checkpoint inhibitors’ importance in lung cancer therapy and advancing immunotherapy strategies in the United States.
In 2026, the immune checkpoint inhibitors market will be exceptionally popular in China because more people are getting cancer, the government is working to improve cancer care, and the healthcare system is getting expanding. increasing investment into clinical trials, more patients being able to access them, and the use of precision medicine are all driving rapid growth and market penetration across the country.
For instance, in January 2026, Henlius, which is based in Shanghai, got IND approval in China for HLX43, a PD-L1-targeting antibody-drug conjugate, along with serplulimab (anti-PD-1) and HLX07 (anti-EGFR) to treat advanced solid tumors. This strategy combines immune checkpoint blockade with targeted therapy to improve effectiveness and get around resistance in hard-to-treat cancers.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 2,369.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 13.2% | 2033 Value Projection: | USD 5,615.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Bristol-Myers Squibb Company, Merck & Co., Inc., F. Hoffmann-La Roche AG, AstraZeneca Plc., Novartis International AG, ImmunOs Therapeutics AG, Immutep Ltd., NewLink Genetics Corporation, Ono Pharmaceutical Co., Ltd., and Pfizer, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The Immune Checkpoint Inhibitors Market growth is due to a growing number of individuals around the world are getting cancer, especially melanoma, non-small cell lung cancer, and renal cell carcinoma. These therapies have shown better results than standard treatments, with longer survival rates and longer-lasting responses. As the number of cancer cases around the world keeps going up, healthcare systems and drug companies are putting a greater focus on ICIs, which is making them more popular. This growing number of patients directly increases revenue and speeds up innovation in the field of oncology.
The shift toward precision oncology and therapies based on biomarkers is greatly increasing the immune checkpoint inhibitors market demand. ICIs can be customized for each patient, which leads to better treatment outcomes and fewer side effects. Doctors are now able to identify the patients who are most likely to benefit from ICIs due to better genetic testing and companion diagnostics. This individualized strategy not only enhances clinical results but also fortifies the market's status as a fundamental element of contemporary cancer treatment, fostering enduring demand across various healthcare environments.
Combining immune checkpoint inhibitors (ICIs) with chemotherapy, targeted therapies, or other immunotherapies is a game-changing chance in cancer treatment. These combinations make treatments more effective, lower the chance of tumors becoming resistant, and improve patients' chances of survival compared to single treatments. As clinical trials keep proving these regimens to be effective, they are likely to be used more widely for many types of cancer. This trend is a key factor in shaping the immune checkpoint inhibitors market forecast. It shows that there is room for growth because of new ideas, more uses, and more patients being able to get them around the world.
The immune checkpoint inhibitors (ICI) market continues to expand rapidly in both clinical and commercial settings, due to increasing uses and acceptance across many types of cancer. Recent reports from the industry show that sales of approved ICIs around the world have reached tens of billions of dollars, reflecting they are becoming a standard part of cancer treatment. The most commonly used drugs are those that target PD-1 and PD-L1 pathways, driven by extensive label expansions and established clinical benefits in diseases like melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, and urothelial carcinoma. Several CTLA-4 inhibitors are still important parts of combination strategies, especially for tumors where monotherapy has limited efficacy.
Volume data from oncology treatment registries indicate that PD-1/PD-L1 inhibitors rank among the most often used systemic therapies for advanced NSCLC, with adoption rates markedly surpassing those of numerous conventional cytotoxic regimens. Long-term survival trends linked to ICI therapy in melanoma have changed the standard of care to include early use, which has had an effect on treatment sequencing around the world.
Pipeline progression is still a major factor, with many investigational targets (like LAG-3 and TIGIT) moving through late-stage development and combination trial programs. New clinical data show that combining new immune modulators with existing PD-1/PD-L1 checkpoint inhibitors may have an extra benefit, especially for tumor types that have not responded to monotherapy in the past.
Cost and access dynamics continue to influence treatment decisions globally, as payers and health systems evaluate long‑term outcomes against therapy expenditure. Increasingly, real-world evidence is being used to improve patient selection and make value-based care approaches work better. The immune checkpoint inhibitors market is growing steadily, with new products and services being added all the time. It is also becoming more integrated into multidisciplinary oncology care pathways.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients