Implantable drug delivery devices are used to administer drugs and fluids without the repeated insertion of needles. This product avoids the need for patients to be hospitalized for intravenous infusion. Implantable drug delivery devices can be aptly utilized for delivery of drugs. Over the last decade, there has been increasing convergence between drug therapies and implantable devices, which include devices such as osmotic pumps and implantable rods that deliver drugs on specific targeted area in the body such as under the skin, intra-vaginal, etc.
Global Implantable Drug Delivery Devices Market - Impact of the Coronavirus (COVID – 19) Pandemic
According to the World Heart Federation, 2020, older people over the age of 60 years and people suffering from underlying medical conditions such as cardiovascular disease, hypertension, diabetes, chronic respiratory disease, and cancer are more prone to the coronavirus infection than the younger population. Furthermore, according to World Health Organization (WHO), on April 2, 2020, it was reported that in Europe, 95% of the coronavirus infection was recorded in the population older than 60 years, while more than 50% of the infection were recorded in the population of the age group of 80 years and more.
In order to protect the vulnerable population from coronavirus infection, people should stay away from all the containment zones including hospitals. This can be possible by implanting drug delivery devices in the patients, which will add an advantage of not personally visiting the hospital for their follow-ups or treatment procedures, as the implantable drug delivery devices offer long term drug delivery. Thus, the COVID – 19 pandemic is expected to spur the global implantable drug delivery devices market growth over the forecast period.
The global implantable drug delivery device market is estimated to be valued at US$ 12,015.2 million in 2020 and is expected to witness a robust CAGR of 7.7% over the forecast period (2020 - 2027).
Figure 1: Global Implantable Drug Delivery Devices Market Share (%) Analysis, By Product Type, 2020
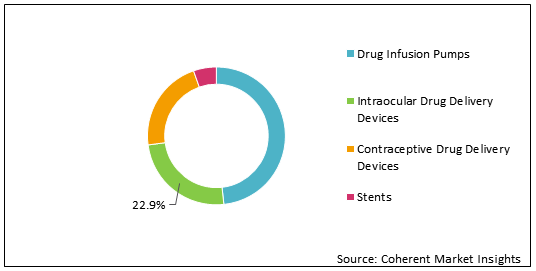
To learn more about this report, Download Free Sample
Increase in acceptance and patient compliance for implantable drug delivery devices is expected to propel the market growth
Implantable drug delivery devices offer several advantages over conventional oral or parenteral dosage form such as protection against gastrointestinal tract denaturation and higher absorption across the wall of intestine or stomach. Secondly, the amount of drug reaching the target should be large to obtain the desired therapeutic effect. Another factor that increases acceptance and patient compliance over traditional mode of administration of drug is the biodegradable systems, which has gained momentum over non-biodegradable delivery system due to the fact that inert polymer used for the fabrication of the device eventually gets absorbed and excreted by the body, alleviating the need for surgical removal of implant after therapy. These factors are expected to propel the market growth over the forecast period.
Increasing global burden of non-communicable diseases is expected to propel growth of the implantable drug delivery devices market
According to a report by World Health Organization (WHO), 2017, chronic diseases are the major cause of disabilities, worldwide. The estimated global burden by 2020 is 60%. Moreover, 73% of deaths in 2020 is expected to be due to chronic diseases. Four of the most prominent chronic diseases are cardiovascular, cancer, pulmonary, and type 2 diabetes.
Implantable drug delivery devices offer unique patient solutions for chronic diseases. For instance, in 2019, Flowonix Medical, Inc. received the U.S. Food and Drug Administration (U.S. FDA) approval for its Prometra II 40 mL Programmable Pump and its associated programmer software. Prometra II 40 mL Programmable Pump is utilized for the intrathecal infusion of Infumorph (preservative-free morphine sulfate sterile solution) or preservative-free sterile 0.9% saline solution (Sodium Chloride Injection, USP) to the cancer patients in order to reduce the pain.
Implantable Drug Delivery Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2019: | US$ 12,015.2 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 7.7% | 2027 Value Projection: | US$ 21,778.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Boston Scientific Corporation, Abbott Laboratories, Medtronic Plc., Merck & Co., Bausch & Lomb Inc., Allergan, Inc., Bayer Healthcare, Alimera Sciences, Eyepoint Pharmaceuticals, DSM Biomedical, Delpor Inc., Teleflex Incorporated, and 3M Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Implantable Drug Delivery Devices – Restraints
Product recalls are expected to restrain growth of the global implantable drug delivery devices market growth. For instance, in 2019, the U.S. FDA recalled the SynchroMed II systems, which was manufactured by Medtronic Plc. Deaths were reported in the patients who were implanted with the SynchroMed II system due to software and mechanical faults that led to the infusion of more or less amount of the drug than the already set value.
The implantable drug delivery devices are expensive. For instance, on February 24, 2020, it was reported that the price of Supprelin LA implant, which is implanted under the skin for the treatment of central precocious puberty, had a price of US$ 37,300. Supprelin LA releases 65 micrograms of the drug a day (histrelin acetate).
Global Implantable Drug Delivery Devices – Regional Analysis
North America is expected to hold dominant position in the implantable drug delivery devices market, owing to increasing research and development activities taking place in the region. For instance, in 2019, Merck Sharp & Dohme Corporation initiated a clinical trial (Phase I) for evaluating the safety and efficacy of Islatravir (MK – 8591) implant in patients suffering from HIV – 1 infection. It was concluded in Phase -1 that Ialatravir implant provides protection for more than a year from spreading of HIV.
Furthermore, increasing product launches is expected to boost the global implantable drug delivery devices market growth over the forecast period. For instance, in 2017, Abbott Laboratories launched XIENCE everolimus-eluting coronary stent system, with features like a thinner profile, increased flexibility, longer lengths, and small-diameters. The stent supports the patients suffering from coronary artery disease to regain their health and to lead a healthy lifestyle.
Figure 2: Global Implantable Drug Delivery Devices Market Value (US$ Mn) & Y-o-Y Growth (%), 2016 - 2027
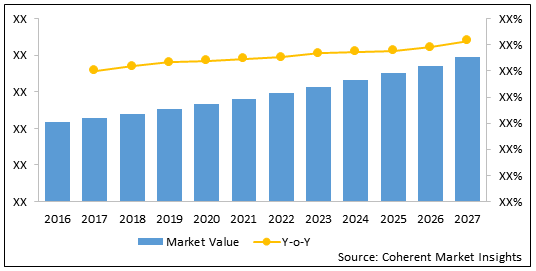
To learn more about this report, Download Free Sample
Global Implantable Drug Delivery Devices Market – Competitive Analysis
Some of the key players operating in the global implantable drug delivery devices market include Boston Scientific Corporation, Abbott Laboratories, Medtronic Plc., Merck & Co., Bausch & Lomb Inc., Allergan, Inc., Bayer Healthcare, Alimera Sciences, Eyepoint Pharmaceuticals, DSM Biomedical, Delpor Inc., Teleflex Incorporated, and 3M Company.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients