Global Injectable Drugs Market is estimated to be valued at USD 614.07 Bn in 2025 and is expected to reach USD 1,032.78 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.7% from 2025 to 2032.
To learn more about this report, Download Free Sample
The rising number of chronic illnesses around the globe is likely to push up demand for injectable medications. Because injections improve bioavailability and deliver drugs quickly through the bloodstream, they are often preferred over pills. Self-injectable options let patients give themselves the shot at home, and developers are now turning preventive treatments into jabs. New delivery technologies keep widening the range of conditions that can be managed with a needle. At the same time, the boom in biologics and large-molecule injectables for cancers, autoimmune disorders, and similar diseases is expected to fuel steady market growth.
|
Event |
Description and Impact |
|
U.S. Tariff Policies Targeting Pharmaceutical Imports |
|
|
Geopolitical Supply Chain Disruptions |
|
|
Drug Shortages and Manufacturing Fragilities |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The global market for injectable drugs is both fast-moving and fiercely competitive, with more than 2,500 active patents currently in force around the world. These cover everything from new formulation chemistry and clever delivery mechanisms to advanced stability-enhancing technologies that extend shelf life. Biologic injectables account for a large share of the estate, and heavyweights like Roche, Amgen, and AbbVie still hold the most coveted rights to key monoclonal antibodies and recombinant protein therapies. Patent filings in device-integrated systems-autoinjectors, prefilled syringes, smart wearable injectors-are equally vigorous, with BD, Ypsomed, and West Pharmaceutical Services at the forefront. Interest in nanoparticle delivery platforms and long-acting depot formulations is quickening, particularly for oncology drugs and treatments aimed at patients with chronic diseases.
The looming patent expirations of blockbuster products such as Humira and Avastin are opening the door for biosimilar injectables, adding fresh pressure in already lucrative therapeutic segments. New applications now regularly cite combination therapies, thermostable biologics, and needle-free devices as core innovations. Taken together, these trends point to a market increasingly defined by drug-device integration, patient-friendly delivery, and next-generation biopharmaceuticals, forces that will steer both competitive tactics and regulatory reviews in the coming years.
The reimbursement framework for injectable medicines varies widely around the world, with each market defined by its own rules, classification buckets, and payer pathways. In the United States, most clinic-administered injectables fall under Medicare Part B, where pricing is anchored to Average Sales Price plus 6 percent, a formula that governs multiple J-coded products-including J9035 for bevacizumab. State Medicaid programs build on this model, mandating deeper rebates yet offering richer rates to low-income enrollees. Commercial payers, in contrast, routinely invoke prior-auth hurdles and list physician-administered injectables under the medical benefit, with overall acceptance rates estimated at 75 to 85 percent. Across Europe, national health-technology assessment bodies such as NICE, IQWiG, and HAS overseen pricing decisions, weighing evidence against pegged willingness-to-pay ceilings and often striking risk-sharing deals that cap budget exposure.
Following European Medicines Agency clearance, each country secures separate pricing and formulary placement, dramatically lengthening time-to-reimbursement if negotiations drift. In the Asia-Pacific zone incentives differ again-Japan employs the Cost-Effectiveness Analysis and Strategic Pharmaceuticals Council model, Australia relies on the Pharmaceutical Benefits Advisory Committee, and China now mandates HTA-and reference pricing driven by the National Medical Products Administration. After a years-long expansion, access improvements persist yet cost-control pressures weigh heavily in many emerging jurisdictions. Taken together, access to injectable drugs hinges on regulatory approval, demonstrated clinical value, and a clear economic rationale, determinants that shape both market penetration and how quickly clinicians adopt innovative therapies.
When doctors choose injectable medicines, they think about how well the drug works, how safe it is, whether patients will keep taking it, the way it is given, and, of course, the overall cost. In oncology, for first-line treatment most experts still reach for IV infusions, so trastuzumab, pembrolizumab, and carboplatin are commonly administered in the clinic or hospital. After initial lines, options widen to include docetaxel, bevacizumab, and emerging therapies such as CAR-T cells, keeping the pipeline moving. Rheumatologists face a different pathway: in early rheumatoid arthritis a subcutaneous shot is simply easier, so adalimumab or etanercept are the usual starters at home.
When the disease does not respond, IV drugs like rituximab and tocilizumab come into play, mirroring the step-up strategy seen in other fields. Neurologists follow a pattern too; with multiple sclerosis interferons and glatiramer acetate work for relapsing episodes, but ocrelizumab or natalizumab step in for progressive forms because they need fewer clinic visits. Across all specialties a clear trend is forming: patients want long-acting injections or products they can give themselves, especially for chronic illnesses that require tight control. Payor rules, updated guidelines, and each patient’s voice still shape the final choice, pushing clinicians toward a more tailored, convenient delivery strategy.
To learn more about this report, Download Free Sample
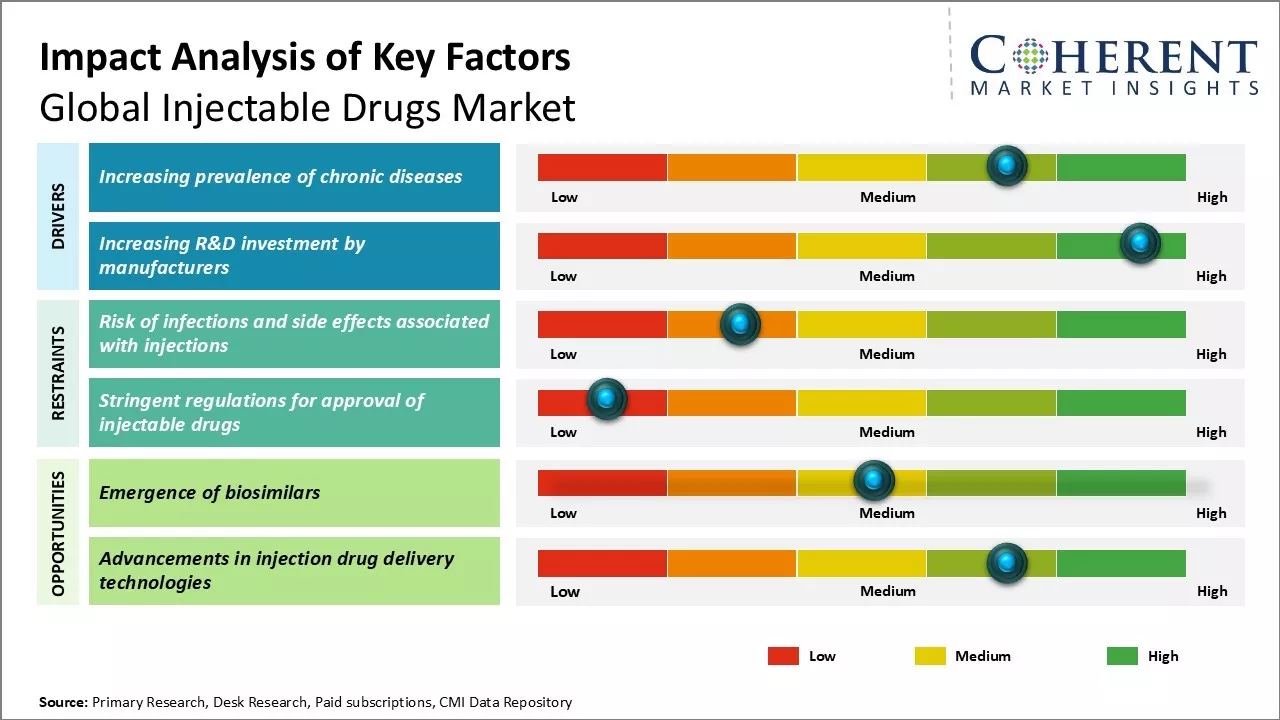
Rising prevalence of chronic diseases can drive the market growth. Chronic diseases such as cancer, diabetes, cardiovascular diseases and respiratory diseases have become one of the major causes of mortality and disability worldwide. According to WHO, chronic diseases accounted for approximately 70% of all deaths globally in 2020. Even in developing countries which were earlier mostly affected by infectious diseases, chronic diseases are increasingly becoming a leading health problem.
Growing burden of chronic diseases poses significant economic challenges for healthcare systems around the world. Treatment of chronic conditions requires long term medication and therapy, which boosts demand for injectable drugs. Many modern drug development approaches are focused on developing drugs which can be self-administered through injections at home. This allows for improved convenience and compliance over oral administration methods.
Chronic diseases also require frequent monitoring of the condition through lab testing and diagnostics. Injectable drugs play a vital role in disease management by allowing targeted and controlled delivery of the required medication directly into systemic circulation. Therapeutic areas like oncology, autoimmune diseases extensively rely on injectables due to their advantages over oral drugs.
Lifestyle changes such as sedentary routines, unhealthy diets and substance abuse have significantly contributed to rise in incidence of chronic medical conditions. Rapidly growing and aging global population susceptible to age-related diseases can increase the prevalence of chronic diseases in the near future.
Unless concrete preventive measures are taken, it is estimated that chronic diseases will remain one of the major health challenges for the foreseeable future. This growing chronic disease burden coupled with limitations of alternative treatment methods has boosted demand for injectable drugs worldwide.
Pharmaceutical companies worldwide have substantially increased their spending on research and development activities over the past few years. Developing new and improved drug delivery mechanisms has been a key priority area. Injectable drug delivery offers several advantages over conventional oral administration such as targeted delivery, sustained release and quicker onset of action. These advantages drive significant R&D efforts towards development of novel and specialized injectable drug formulations.
Modern biologics and large molecule drugs have exponential growth opportunities, however, pose delivery challenges. Injectables provide a viable solution through formulations like pre-filled syringes, auto-injectors and implantable pumps. Substantial investments are dedicated to applying advanced drug formulation and delivery technologies such as microencapsulation, nanoparticle drug carriers and controlled release injectable depots.
Such innovations allow delivery of drugs which were previously considered unsuitable for injection. Complex biologics are increasingly being developed in safer, stable and patient friendly injectable forms.
Moreover, injectable drug digitization is revolutionizing medication management. Combination of drug delivery devices, sensors and connectivity platforms enable development of “Intelligent” injectables with capabilities like automatic dosing, wireless health monitoring. Such “Digital therapeutics” are being co-developed along with traditional injectable drugs to improve clinical outcomes.
Significant R&D spending is focused on such combination product development approaches. Overall advancement in materials, engineering and data science boosts introduction of human centric specialized injectable products with augmented functionalities.
The emergence of biosimilars presents a major growth opportunity for the global injectable drugs market. Biosimilars are biological products that are similar to an already approved biological reference product known as the originator biologic. As patents of many blockbuster biologics expire, multiple pharmaceutical companies are developing biosimilars of those products. This increases treatment options for patients and physicians apart from lowering healthcare costs significantly compared to originator biologics.
The uptake of affordable biosimilars is expected to be faster in the injectable segment as parenteral administration remains the preferred route for many monoclonal antibody drugs and other biologics. The biosimilars market is projected to grow substantially in the near future, thus, creating abundant opportunities for injectable drug manufacturers. This can enhance access to lifesaving therapies and propel the expansion of the worldwide injectable drugs market.
By molecule type, small molecule segment is estimated to contribute the highest market share of 59.7% in 2025, owing to its affordability. Small molecule drugs have been the mainstay of treatment for various therapeutic areas due to their affordability and ease of production. Being composed of relatively smaller organic compounds, small molecule drugs have low production costs as compared to large molecule biologics. Their simple structure also makes them suitable for generic development, further, enhancing access and reducing costs for patients worldwide.
A major factor driving the use of small molecule drugs is their prevalence in areas like oncology, cardiovascular diseases and anti-infectives, which account for the bulk of disease burden. Many first line treatments and standard of care regimens in these therapeutic segments rely on small molecule formulations.
For instance, drugs like paclitaxel, docetaxel and oxaliplatin constitute the first line chemotherapeutic regimens in breast, lung and colorectal cancers. Antibiotics, anti-hypertensives and statins form the mainstay of infectious disease, cardiovascular and endocrine treatments globally.
The off-patent status of many important small molecule drugs also promotes their affordability and widespread prescribing. The availability of low cost generic versions of blockbuster drugs like atorvastatin, enoxaparin and sunitinib has transitioned treatment to the generic small molecule counterparts. Biosimilars have captured a sizeable share of markets of off-patent blockbuster biologics, thus, shifting treatment paradigms.
Ease of administration through the parenteral route including intravenous, intramuscular and subcutaneous injections has further increased the acceptability of small molecule drugs. While large molecules often require specialized delivery methods, small molecules can be formulated into ready-to-use vials, pre-filled syringes and intravenous infusions to facilitate outpatient and ambulatory care. This supports treatment convenience and adherence.
Strong cost advantages, prevalence in high volume disease areas and supportive intellectual property status have cemented the leading position of the small molecule segment in the global injectable drugs market.
By route of administration, intravenous segment is estimated to contribute the highest market share of 40.5% in 2025, due to its effectiveness. Intravenous therapy witness widespread adoption due to its effectiveness in delivering drugs systemically. Being the most direct non-oral administration method, intravenous injections deliver drugs to the bloodstream immediately after administration. This allows rapid achievement of therapeutic drug concentrations without reliance on gastrointestinal absorption.
This is a major advantage in several clinical situations like emergency treatment of conditions including infections, cardiac events, hemorrhagic shock and even cancer. Timely systemic delivery of drugs is critical in these life-threatening conditions requiring immediate clinical response. The effectiveness of intravenous route has made it the preferred way of drug administration in hospitalized patients and critical care.
Intravenous injections are also useful for drugs that are either not absorbed or inactivated after oral/enteral administration. Examples are antibacterials, antifungals, chemotherapeutics, anticoagulants and some parenteral nutrition formulas. Direct venous access ensures the entire bioavailable dose reaches the systemic circulation from first pass metabolism or gastrointestinal degradation.
From a patient standpoint, intravenous therapy enables non-oral administration for those unable to take medications orally - like unconscious or vomiting patients. It is also utilized for conditions requiring controlled and precise delivery of drugs on fixed schedules, as the rate and amount injected can be carefully regulated.
The technical expertise required in administering intravenous injections through cannulated veins is a drawback limiting its use to hospital-based and skilled ambulatory care. However, high efficacy outcomes has ensured intravenous route maintains its primacy in the global injectable drugs market over others like intramuscular or subcutaneous.
By therapeutic area, Oncology segment is estimated to contribute the highest market share of 35.5% in 2025, due to escalating incidence rates of cancer worldwide. Cancer has replaced cardiovascular diseases as the leading cause of death globally, responsible for nearly 10 million lives lost in 2020. Growing aging population prone to cancers, adoption of lifestyle changes and other environmental risk factors have all contributed to the tremendous rise in new cancer cases annually across all regions.
Multimodality treatment approach in oncology also drives the segment growth. Most cancer regimens involve a combination of surgery, radiation, chemotherapy and newer targeted or immunotherapy agents. Among these modalities, injectable chemotherapy in the form of intravenous or subcutaneous infusions represents the mainstay of treatment for majority of cancer types and stages worldwide. The permanence of chemotherapy also boosts use of these drugs.
Biologics constitute a notable share of novel oncology treatments now available. These injectable monoclonal antibody and immune-checkpoint inhibitors play the role of first line therapy or successors to chemotherapy. Examples include Avastin, Keytruda and Opdivo with multi-billion dollar annual sales worldwide. Furthermore, biologics and biosimilars approvals can sustain new injectable additions in oncology.
Geographical expansion of cancer care can also drive the segment growth. Economic development of Asian and African countries is improving access to sophisticated care like injectable chemotherapy and targeted therapies previously limited to developed markets. This widening treatment availability boosts injectable oncology drugs market volume internationally. Heightened cancer screening and focus on reduced out of pocket costs of treatment can aid penetration globally.
To learn more about this report, Download Free Sample
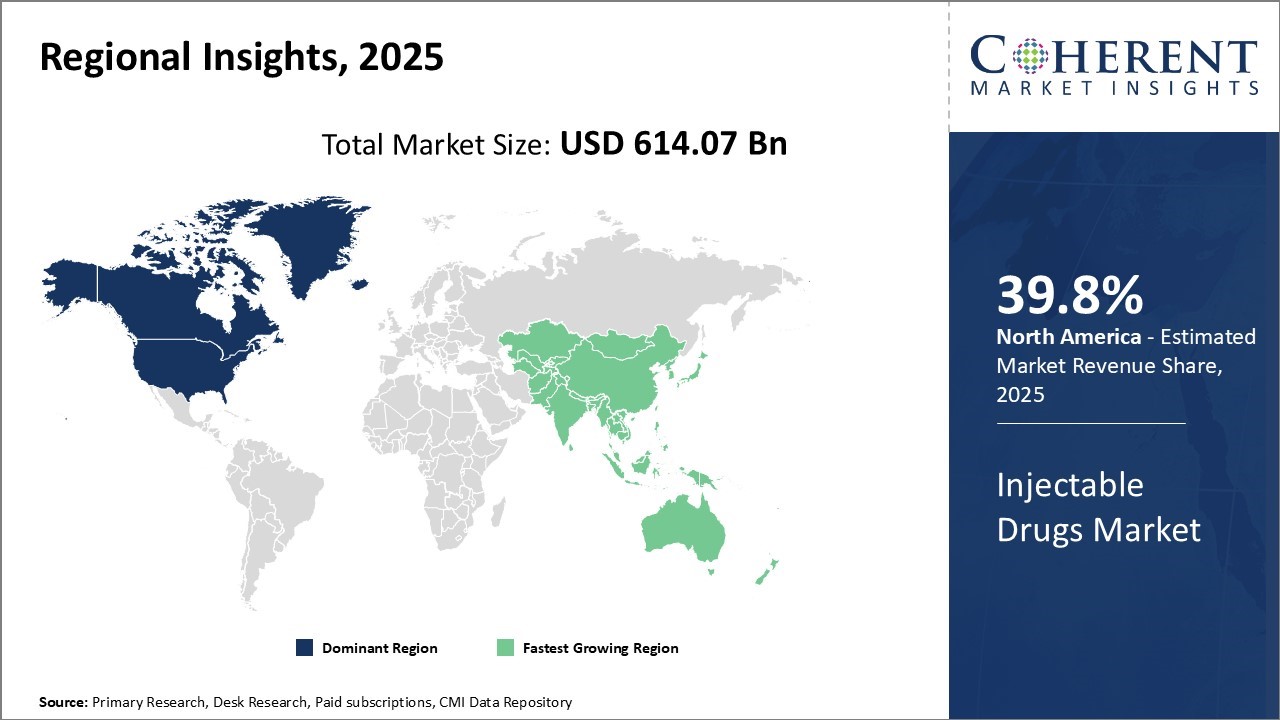
North America is poised to dominate the global injectable drugs market, capturing an estimated 39.8% market share in 2025. This leadership is driven by the strong presence of major pharmaceutical companies in the United States, which serve as global hubs for R&D, manufacturing, and innovation. The region benefits from advanced research infrastructure, favorable regulatory policies, and close collaboration between industry and academia.
High healthcare expenditure, along with a growing aging population that demands treatment for chronic illnesses such as cancer and diabetes, further accelerates the use of injectable therapies. Additionally, minimal price controls and solid intellectual property protections enable pharmaceutical companies to sustain innovation and maintain premium pricing.
Asia Pacific is projected to be the fastest-growing regional market for injectable drugs, supported by its large patient base and the rising burden of both communicable and non-communicable diseases. Countries like China and India are at the forefront of this growth, owing to their significant role in global pharmaceutical manufacturing and increasing local demand.
The region is experiencing rapid healthcare infrastructure improvements and government-backed initiatives to enhance domestic production through import substitution strategies. Rising urbanization, improved access to healthcare services, and increased foreign investments from multinational pharma companies are expanding market accessibility and affordability across Asia Pacific.
The United States is the dominant player in the North American injectable drugs market, backed by a well-established pharmaceutical industry, a high prevalence of chronic diseases, and extensive healthcare coverage. The country’s advanced clinical trial network, regulatory clarity from the FDA, and strong investment in drug development provide a fertile ground for innovation in injectable formulations. Widespread adoption of specialty biologics and biosimilars further reinforces its leadership position.
China is a key growth engine in the Asia Pacific region, benefiting from its large aging population, government reforms to boost healthcare access, and expanding domestic manufacturing capabilities. Recent initiatives promoting innovation, biosimilar approvals, and reforms to fast-track drug approvals are encouraging global firms to invest in China’s injectable drug landscape. The country’s dual strategy of import reduction and export growth is strengthening its role in the global supply chain.
India represents a rapidly expanding injectable drugs market, supported by a robust generics manufacturing base and rising healthcare investments. The government's focus on Atmanirbhar Bharat (self-reliant India) has incentivized local production of injectables, while the pharmaceutical sector benefits from skilled labor and competitive production costs. With increasing public and private healthcare expenditures, India is emerging as both a major exporter and a high-demand market for injectable therapeutics.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 614.07 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.7% | 2032 Value Projection: | USD 1,032.78 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Teva Pharmaceutical Industries Ltd., Sanofi S.A., Sun Pharmaceutical Industries Ltd., AstraZeneca Plc, Merck & Co., Inc., Viatris + Mylan N.V, Cipla Inc., Dr Reddy’s Laboratories Ltd., Samsung Biologics, Abbott Laboratories, Amgen Incorporated, Baxter International Incorporated, Becton Dickinson and Company, Bristol-Myers Squibb Company, GlaxoSmithKline Plc, Roche Holding Limited |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global injectable drugs market encompasses pharmaceutical medications designed for administration through needle-based injections or infusions into patients. This market includes a variety of common injectable drugs, such as vaccines, insulin, antibodies, chemotherapeutic agents, and analgesics. These products are administered via different routes, including intravenous, intramuscular, and subcutaneous injections, and are used in both chronic disease management and acute treatments within hospitals and clinics.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients