Lentiviral Vectors Market is estimated to be valued at USD 410.0 Mn in 2025 and is expected to reach USD 1,345.3 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 18.5% from 2025 to 2032.
Analysts’ Views on Global Lentiviral Vectors Market:
Over the projected period, the market is anticipated to be driven by growing research & development activities by market participants and growing partnerships and license agreement by the market key players. For instance, in June 2021, National Resilience, Inc., a technology-focused biomanufacturing company, and bluebird bio, Inc., a biotechnology company that develops gene therapies for severe genetic disorders, announced a strategic alliance aimed to accelerate the early research, development, and delivery of cell therapies. As part of the agreement, Natural Resilience, Inc., will acquire bluebird’s Research Triangle (bRT) manufacturing facility located in U.S and retain all of the more than 100 highly skilled technical staff and administrators currently employed at the site. Natural Resilience, Inc., will continue to support vector supply for both bluebird bio and 2seventy bio, Inc. The two companies are also finalizing a definitive agreement to establish partner programs that will share expense and revenue for successful commercialized oncology products and in parallel establish a next-generation manufacturing research & development collaboration.
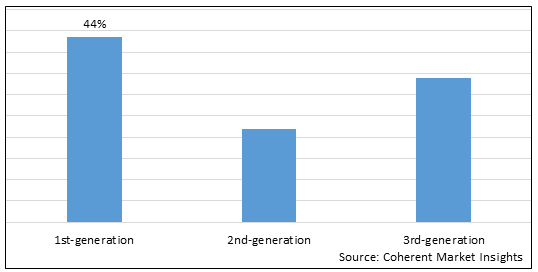
Figure 1. Global Lentiviral Vectors Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
Global Lentiviral Vectors Market– Drivers
Increasing licensing agreement by the market key players
Increasing licensing agreement by the market key players is expected to drive the market over the forecast period. For instance, in October 2020, Mustang Bio, Inc., a clinical-stage biopharmaceutical company, and Sirion-Biotech GmbH, a biotechology company, announced a licensing agreement under which Mustang Bio, Inc. has acquired rights to SIRION’s LentiBOOST technology for the development of MB-207, Mustang’s lentiviral gene therapy for the treatment of patients with X-linked severe combined immunodeficiency (“XSCID”), also known as bubble boy disease, who have been previously treated with a hematopoietic stem cell transplantation (“HSCT”) and for whom re-treatment is indicated. LentiBOOST is SIRION’s proprietary non-cytotoxic transduction enhancer for lentiviral vectors
Increasing collaboration agreement between the market key players
Increasing collaboration agreement between the market key players is expected to drive the market over the forecast period. For instance, in April 2021, Albumedix Ltd., a science-driven company, has announced an extension to its research collaboration with Cobra Biologics, the gene therapy division of Cognate BioServices, a Charles River Laboratories International, Inc. company. The research collaboration focuses on process optimization and stability enhancement in scale-up manufacturing of AAV (adeno-associated virus) and lentiviral vectors through Albumedix' proprietary recombinant human albumin-based product.
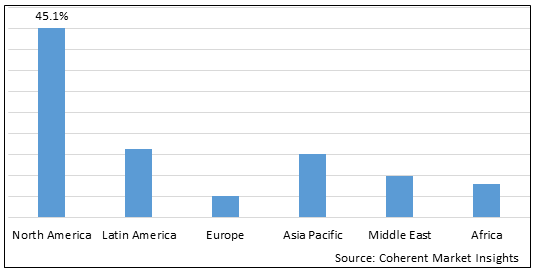
Figure 2. Global Lentiviral Vectors Market Value (US$ Million), By Region, 2025
To learn more about this report, Download Free Sample
Global Lentiviral Vectors Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global lentiviral vectors market over the forecast period owing to the increase in license agreement and collaboration by market key players and increasing prevalence of HIV. For instance, on June 21 2025, according to the Centers for Disease Control and Prevention, in the U.S., an estimated 1.2 million people were living with HIV as of the end of 2021, and about 87% of them were aware that they had HIV.
Global Lentiviral Vectors Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global lentiviral vectors market, owing to the demand for the vaccines to treat covid-19 disease. For instance, in February 2021, according to an article published by the Public Library of Science (PLOS), due to COVID-19 pandemic, many laboratories have redirected attention to SARS-CoV-2, indicating that there is a pressing need for equipment that can be used in labs that are unfamiliar with working with coronaviruses, which has prompted research into the COVID-19 treatment. This increases the demand for lentiviral vectors for the treatment of COVID-19 disease.
Global Lentiviral Vectors Market- Segmentation
The global lentiviral vectors market report is segmented into product type, indication, end user, and region.
Based on Product Type, the global lentiviral vectors market is segmented into 1st-generation, 2nd-generation, and 3rd-generation. Out of which, the 1st-generation segment is expected to dominate the market during the forecast period and this is attributed because it is used to treat a variety of disorders The gag and pol genes, as well as a number of other viral proteins, are present in first-generation lentiviral vectors in substantial amounts. So, key players are focusing on strategies such as collaboration as well as acquisition for the global lentiviral vectors market growth.
Based on Indication, the global lentiviral vectors market is segmented into HIV, β-thalassemia, X-linked adrenoleukodystrophy, metachromatic leukodystrophy, and Wiskott- Aldrich syndrome. The HIV segment is expected to dominate the market over the forecast period owing to the increasing prevalence of HIV and increasing demand to treat it.
Based on End User, the global lentiviral vectors market is segmented into hospitals, clinics, and research institutes. The research institutes segment is expected to dominate the market over the forecast period due to increasing initiative activities by various healthcare organizations and government bodies to encourage research endeavor.
Based on Region, the global lentiviral vectors market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which, the North America is expected to dominate the market over the forecast period owing to the increase in the prevalence of HIV patients as well as license agreement and collaboration by market key players.
Among all segmentation, the product type segment has the highest potential due to the increasing partnership as well as acquisition by the key market players over the forecast period to develop the novel therapy for HIV, β-thalassemia, and X-linked adrenoleukodystrophy. On June 07 2023, Orgenesis Inc., a global biotech company working to unlock the full potential of cell and gene therapies (CGT), and a U.S-based University of California, announced an agreement to roll out proprietary Orgenesis Mobile Processing Units and Labs at medical or academic institutions within the University of California (UC) system. The partnership agreement sets out a staged approach through which Orgenesis will install and operate OMPULs(TM), enabling its proprietary point-of-care (“POCare”) Service Platform to manufacture therapeutics at hospitals throughout California, empowering onsite production for clinical trials like the cell and gene therapies in development at UC Davis Health’s Alpha Stem Cell Clinic.
Global Lentiviral Vectors Market- Cross Sectional Analysis
Among indication, the HIV segment held a dominant position in North America region over the forecast period owing to the increasing prevalence of HIV and increasing demand to treat it. For instance, in October 2022, according to the HIV.gov, Approximately 1.2 million people in the U.S. have HIV and about 34,800 people were newly infected with HIV in the U.S. in 2020.
Global Lentiviral Vectors Market- Key Developments
On February 07 2023, Genenta Science, a clinical-stage biotechnology company engaged in the development of hematopoietic stem progenitor cell immuno-gene therapy for cancer, has entered into a development and manufacturing service agreement (MSA) with AGC Biologics, a global Contract Development and Manufacturing Organization to manufacture cell therapy lentivirus-based product for Genenta's ongoing clinical programs. AGC Biologics offers end-to-end global viral vector and cell therapy development, manufacturing and quality/regulatory services, supported by scientists with 30 years of knowledge and experience.
In July 2022, Oxford Biomedica, a gene and cell therapy company specialising in the development of gene-based medicines, announced that it has signed a new Licence and Supply Agreement (LSA) with an undisclosed U.S.-based private biotechnology company advancing a new generation of adoptive cell therapies. The LSA grants the new partner a non-exclusive licence to utilise Oxford Biomedica’s LentiVector platform for its application in their lead (chimeric antigen receptors-therapy) CAR-T programme, and puts in place a three-year Clinical Supply Agreement. Under the terms of the LSA, Oxford Biomedica will receive an undisclosed upfront payment, as well as additional payments related to the development and manufacturing of lentiviral vectors for use in clinical trials. The Company will also receive certain development and regulatory milestone payments and an undisclosed royalty on the net sales of products sold that utilise the Company’s LentiVector platform.
In November 2022, ReiThera srl, a biotech company dedicated to the development of new technologies, (good manufacturing practices) GMP production, and the clinical translation of genetic vaccines and products for advanced therapies, announced that it has received operational authorization from the Italian Medicines Agency (AIFA) to open the new production area at its pharmaceutical facility at the Castel Romano Technopole, for the large-scale production of viral vectors for vaccines and gene therapy.
In May 2022, The Janssen Pharmaceutical Companies of Johnson & Johnson announced that the European Commission (EC) granted conditional marketing authorization of CARVYKTI for the treatment of adults with relapsed and refractory multiple myeloma (RRMM) who have received at least three prior therapies, including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI) and an anti-CD38 antibody, and have demonstrated disease progression on the last therapy.
Global Lentiviral Vectors Market- Key Trends
Increasing prevalence of chronic diseases such as diabetes, etc.
Increasing prevalence of chronic diseases such as diabetes is expected to fuel the market over the forecast period owing to the application of gene therapy which improve glycemic control and reduce the need for insulin therapy. For instance, on April 05 2023, according to the (WHO) World Health Organization, in 2020, diabetes was the direct cause of 1.5 million deaths and 48% of all deaths due to diabetes occurred before the age of 70 years globally.
Lentiviral Vectors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 410.0 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 18.5% | 2032 Value Projection: | USD 1,345.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Thermo Fisher Scientific Inc., Sirion-Biotech GmbH (Revvity), Vector Biolabs, OriGene Technologies, Inc., SignaGen Laboratories, Sino Biological, Inc., Takara Bio Inc., Cell Biolabs, Inc., GenTarget Inc., GENEMEDI, bluebird bio, Inc., Cellomics Technology, LLC., Virica Biotech, Oxford Biomedica, and ANDELYN BIOSCIENCES. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Lentiviral Vectors Market: Restraints
The possibility of oncogenesis through insertional mutagenesis
The possibility of oncogenesis through insertional mutagenesis is expected to hinder the market growth over the forecast period. So, in-vitro methods such as irradiation and treatment with chemical mutagens of inducing mutation cane be employed. For instance, in March 2021, according to an article published in the Journal of Cancer Molecular targets and Therapeutics, published by the Frontiers, the risk of inserting a gene into a tumor suppressor gene or activating an oncogene is present for the vectors that integrate into the unwanted locations of the genome, such as retrovirus. To counter this, vectors can be used that do not integrate readily into the genome. Additionally, self-inactivating vectors can be manufactured that do not contain their own promoter; rather, another internal promoter in the cell is used. This leads to less genotoxicity and is a safer alternative to traditional integrating vectors.
Global Lentiviral Vectors Market- Key Players
Major players operating in the global lentiviral vectors market include Thermo Fisher Scientific Inc., Sirion-Biotech GmbH (Revvity), Vector Biolabs, OriGene Technologies, Inc., SignaGen Laboratories, Sino Biological, Inc., Takara Bio Inc., Cell Biolabs, Inc., GenTarget Inc., GENEMEDI, bluebird bio, Inc., Cellomics Technology, LLC., Virica Biotech, Oxford Biomedica, and ANDELYN BIOSCIENCES.
Global Lentiviral Vectors Market– Definition
Lentiviral vectors are used as a means to deliver foreign genetic material into another cell. These vectors are developed from lentiviruses, a type of retrovirus, which are characterized by their long incubation period and can infect both dividing and non-dividing cells. The lentiviral vectors are derived from the human immunodeficiency virus (HIV), and hence, these vectors are highly efficient vehicles for in vivo gene delivery for gene therapies.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients