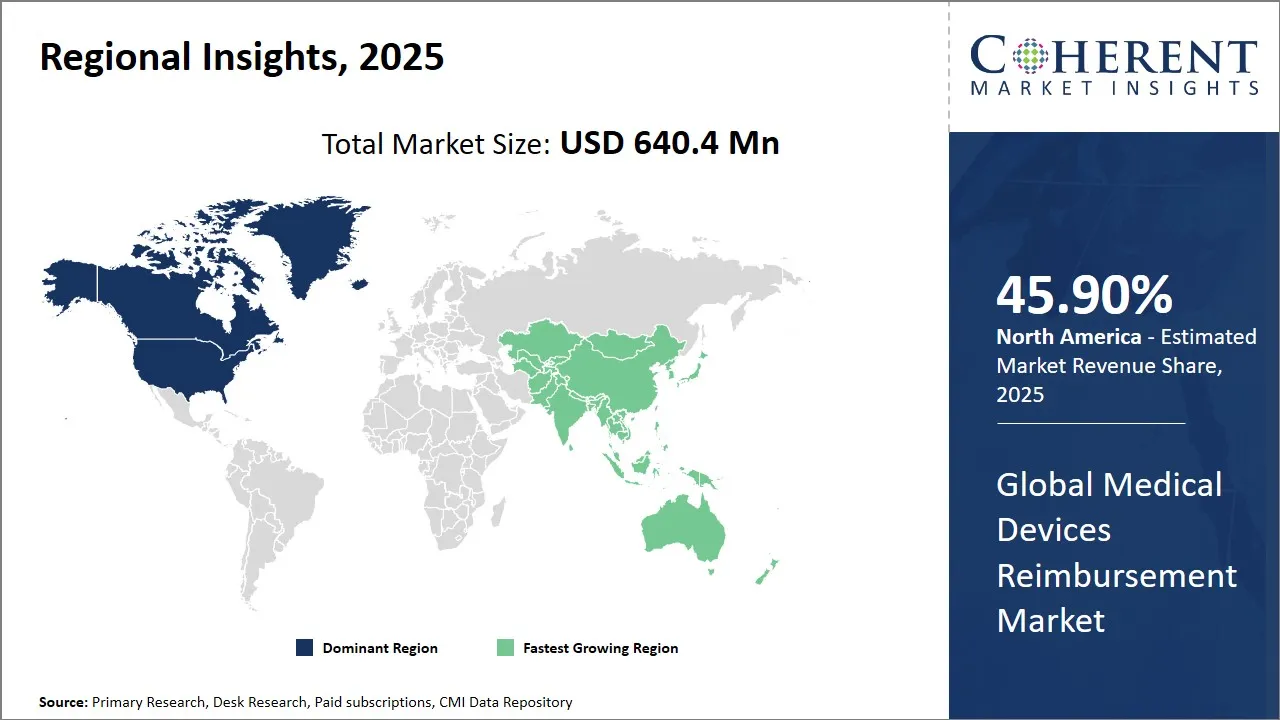
Medical Devices Reimbursement Market is estimated to be valued at USD 640.4 Mn in 2025 and is expected to reach USD 1,140.9 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.6% from 2025 to 2032.
The medical devices reimbursement market demand is rising due to increasing chronic diseases, aging populations, and growing healthcare costs. Supportive government initiatives and reimbursement programs are boosting access to advanced devices. However, complex regulatory frameworks and limited coverage for newer technologies remain key barriers. Despite these challenges, ongoing reforms and value-based care models are expected to drive market expansion globally.
|
Current Event |
Description and Its Impact |
|
U.S. Medicare Reimbursement Changes |
|
|
EU Medical Device Regulation (MDR) Compliance Pressures |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The integration of artificial intelligence into medical devices reimbursement market is increasingly transforming by accelerating approval processes and by aligning devices with modern value-based care models. As healthcare systems shift toward outcomes-based reimbursement, AI-enabled medical devices, such as diagnostic imaging tools, wearable monitors, and predictive analytics systems are gaining traction because they offer real-time data, early disease detection, and better patient management.
AI-driven devices provide measurable clinical outcomes through continuous monitoring and predictive alerts (e.g., AI-powered ECG or glucose monitoring), making it easier for payers to justify coverage under value-based reimbursement models.
Moreover, Payers and manufacturers use AI to predict health outcomes and economic impact more accurately. This supports coverage decisions and pricing negotiations with health insurers or national health systems.
In March 2025, AdvaMed, the MedTech Association released its AI Policy Roadmap, a strategic framework aimed at guiding Congress and federal agencies, including the FDA and CMS, through the emerging era of AI-enabled medical technologies. The roadmap identifies three key priority areas: privacy and data access, an evolved FDA AI regulatory framework, and reimbursement and coverage policies.
In terms of payers, the private segment is expected to hold 65.9% of the market share in 2025, due to their focus on cost-efficiency and improving patient outcomes. As the demand for advanced and high-cost medical technologies grows, especially in areas like diagnostics, home monitoring, and minimally invasive devices, private insurers are seeking ways to manage healthcare expenditures while ensuring access to effective treatments. They are also promoting value-based reimbursement models that tie payments to clinical effectiveness, patient satisfaction, and long-term cost savings. This shift reflects their growing role in shaping coverage policies and incentivizing the use of innovative, cost-effective medical devices.
In July 2024, Star Health insurance launched its home healthcare services in 50 Indian cities and promises to provide customers with 100% cashless in-home medical care for various infectious disease via the Star Health Mobile App. This new launch is highly personalized, customer-centric and aims to provide effective healthcare solution at customer’s doorstep.
In terms of healthcare settings, the hospitals segment is expected to contribute the highest share of the market in 2025, due to their high patient volume and reliance on advanced diagnostic and therapeutic technologies. They often handle complex procedures that require reimbursable high-cost devices such as cardiac implants, surgical robotics, and monitoring systems. Additionally, hospitals actively engage with public and private payors to ensure coverage, making reimbursement policies crucial to their operations and budgeting. Reimbursement enables hospitals to adopt new technologies without passing excessive costs to patients, thereby improving patient outcomes and financial sustainability. This is further propelling the medical devices reimbursement market revenue.
In June 2025, BESLER, a market leader in revenue recovery and hospital reimbursement solutions, introduced BESLER OMNIA, the industry’s first comprehensive platform that enables healthcare organizations to seamlessly prepare and submit both 2552-10 and 287-22 Medicare Cost Reports in a single, secure solution.
To learn more about this report, Download Free Sample
North America region’s dominance in the global medical devices reimbursement market, with an estimated share of 45.90% in 2025, is driven by a well-established healthcare infrastructure, high healthcare expenditure, and a favorable reimbursement environment. Government programs like Medicare and Medicaid in the U.S. provide extensive coverage for a wide range of medical devices, encouraging both usage and innovation. Additionally, the region's high prevalence of chronic diseases and aging population fuels demand for long-term and home-based medical technologies, further boosting the medical devices reimbursement market share. Frequent policy updates and strong presence of private insurers also contribute to a dynamic and evolving reimbursement ecosystem in North America.
For instance, in April 2025, Bipartisan senators introduced the Health Tech Investment Act, which would establish a Medicare reimbursement pathway for FDA-cleared medical devices that utilize artificial intelligence and machine learning. The Secretary of Health and Human Services will collect data through algorithm-based devices to a new technology ambulatory payment classification (APC), which will be determined based on cost data from the service manufacturer. Such data would include invoice prices, subscription-based fees, overhead costs, clinical staff expenses and other costs associated with providing service.
The Asia Pacific region is expected to exhibit the fastest growth in the medical devices reimbursement market during the forecast period. The expansion is observed due to growing healthcare infrastructure, rising insurance penetration, and growing government support for universal health coverage. Countries like China, India, and Japan are witnessing a surge in chronic conditions, which is increasing demand for reimbursable medical devices. Additionally, public health schemes like India’s Ayushman Bharat and Japan’s universal insurance model are helping drive market adoption by improving affordability and access to advanced diagnostics and treatments. The region’s large population and fast-growing middle class further contribute to strong market potential.
In January 2025, the administration of Pt. Bhagwat Dayal Sharma University of Health Sciences (UHS) set up a specialized Medical Reimbursement Cell. This initiative aims to manage reimbursement cases efficiently and ensure prompt payment of medical expenses for employees.
The United States leads the medical devices reimbursement market due to its well-established systems like Medicare and private insurance. The growing focus on value-based care supports coverage for devices that improve outcomes and reduce costs. High demand exists for durable medical equipment (DME), home healthcare devices, and continuous glucose monitors (CGMs). Policy support for remote monitoring and faster reimbursement approvals is further driving market growth.
Japan remains a key player in the medical devices reimbursement market due to its universal healthcare system and aging population. With over 28% of its citizens aged 65 or older, demand is rising for diagnostic imaging devices, cardiac implants, and orthopedic prosthetics. The government’s structured reimbursement framework, led by the Ministry of Health, ensures predictable pricing and supports the adoption of advanced medical technologies. Japan’s emphasis on cost-effective, high-quality care makes it an attractive market for device manufacturers.
Germany is a key driver in the medical devices reimbursement market due to its strong public healthcare system and structured reimbursement via Statutory Health Insurance (SHI). With a large aging population and focus on high-quality care, there is strong demand for surgical instruments, wound care products, and rehabilitation devices. Clear reimbursement pathways make Germany a favorable and transparent market for medical device manufacturers.
In July 2024, the German Bundestag passed key reforms to drug pricing and reimbursement laws as part of the newly adopted Medical Research Act (Medizinforschungsgesetz, MFG). Introduced by the Federal Health Ministry (BMG), the Act includes amendments to national regulations for clinical trials involving drugs and medical devices, rules for advanced therapy medicinal products (ATMPs), the AMNOG drug pricing and reimbursement framework, and restructures regulatory authorities and ethics committees. This is further accelerating the medical devices reimbursement market demand.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 640.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.6% | 2032 Value Projection: | USD 1,140.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
BNP Paribas, CVS Health, Aviva, Allianz, Humana, Cigna, Aetna, Wellcare Health Plans Inc., UnitedHealth Group Inc., and Nippon Life Insurance Company, among others |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
One of the key factors expected to augment growth of the global medical devices reimbursement market during the forecast period is the increasing prevalence of chronic diseases worldwide. Such a high burden of chronic diseases leads to an increase in demand for affordable and high-quality healthcare services, and it is expected to propel the medical devices reimbursement market growth. According to the World Health Organization (WHO), non-communicable diseases (NCDs) kill over 41 million people each year, equivalent to 74% of all deaths worldwide. Cardiovascular diseases account for most NCD deaths, or 17.9 million people every year, followed by cancers (9.3 million), chronic respiratory diseases (4.1 million), and diabetes (2.0 million), further proliferating the medical devices reimbursement market demand.
Another factor which is driving the growth of the global medical devices reimbursement market is the rise in geriatric (aging) population around the world. For instance, aging population is prone to developing infections and associated complications, which in turn increased need for affordable healthcare services. According to the UN DESA’s (United Nations Department of Economic and Social Affairs) Population Division, 1 in 6 people in the world will be over the age 65 by 2050, up from 1 in 11 in 2019. In many regions, the population aged 65 will double by 2050, while global life expectancy beyond 65 will increase by 19 years.
The future opportunities in the medical devices reimbursement market forecast is expected to witness integration of digital and connected medical devices. As digital health and remote patient monitoring (RPM) grow, reimbursement frameworks will increasingly cover wearables, AI-enabled diagnostics, and telemedicine-compatible devices. Devices like smart insulin pens, ECG monitors, and continuous glucose monitors are becoming essential, and countries are updating coverage plans accordingly. Innovators of connected devices can tap into expanding reimbursement policies that now prioritize value-based care and real-time health data. additionally, manufacturers are focusing on home-based and preventive care due to to aging populations and cost concerns, more governments are funding at-home medical care. Devices like portable oxygen concentrators, home dialysis units, and remote wound care tools are gaining reimbursement traction, which will lead to manufactures capitalizing on this shift by developing or adapting devices for home use, which are eligible for newer reimbursement models.
Reimbursement pathways for digital health solutions are evolving at different speeds in different markets. For instance, in Europe, the Government of Germany, Sweden, and the United Kingdom are promoting the digitization of care and have standardized reimbursement pathways. Germany introduced Digital Healthcare Act in December 2019, which enables physicians prescribe digital health applications to patients and for them to seek reimbursement from healthcare insurers for these apps that meet certain criteria issued by the government. This trend is expected to continue over the forecast period, driving the growth of the market.
The medical devices reimbursement market value has entered a critical inflection point, where the convergence of cost-containment pressures, clinical value demonstration, and payer-provider dynamics is reshaping innovation pathways. While reimbursement mechanisms have traditionally lagged behind technological evolution, the current market climate reveals a maturing yet highly selective framework favoring devices that not only demonstrate clinical efficacy but also deliver quantifiable economic utility within specific care settings.
One of the most pivotal trends shaping reimbursement decisions is the shift toward value-based healthcare models. In leading markets such as Germany, Japan, and the United States, payers are increasingly evaluating devices based on real-world performance rather than controlled trial outcomes alone. For instance, the Centers for Medicare & Medicaid Services (CMS) in the U.S. recently expanded the Coverage with Evidence Development (CED) approach, requiring developers of breakthrough devices to generate ongoing post-market data to sustain coverage. The implication is clear: reimbursement is no longer a fixed outcome; it is a dynamic lifecycle obligation.
This evolution is particularly evident in cardiovascular and diabetes management devices, where reimbursement is increasingly linked to patient adherence and outcome optimization. The French Social Security system, for example, has linked reimbursement for continuous glucose monitoring (CGM) systems such as Abbott’s FreeStyle Libre to documented improvements in glycemic control. This performance-tethered reimbursement mechanism is a blueprint for future therapeutic areas, especially as digital therapeutics and AI-enabled diagnostics blur the boundaries between device and service.
Moreover, the heterogeneity across regional reimbursement systems is no longer a bureaucratic nuisance, it is a strategic lever. Japan’s structured and centralized reimbursement environment, supported by the Chuikyo committee, offers device manufacturers predictability but demands compelling health economic models, often favoring locally generated clinical evidence. In contrast, India’s reimbursement ecosystem remains underdeveloped but presents a greenfield opportunity for managed care penetration through public-private schemes such as Ayushman Bharat. Device makers that tailor market access strategies around such divergence, adapting value dossiers, pricing corridors, and post-market surveillance tools will command an outsized advantage.
From an industry standpoint, one miscalculation continues to surface: the overemphasis on regulatory approval as a gateway to reimbursement. While regulatory clearance ensures market entry, it does little to guarantee payer adoption. Devices that are well-positioned from a clinical standpoint but lack health economic substantiation or fail to integrate with digital health infrastructure often face reimbursement delays, if not outright denials.
*Definition: Medical devices reimbursement is defined as the payment a private insurer pays to healthcare providers for costs the provider incurred while using medical devices.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients