Global primary sclerosing cholangitis market is estimated to be valued at USD 174.1 Mn in 2025 and is expected to reach USD 298.6 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
There is no approved drug to treat primary sclerosing cholangitis and current treatment options are limited to relieving symptoms and managing complications. However, the market is expected to benefit from ongoing research and rising number of clinical trials for developing novel therapeutics for effective treatment of the disease. Increasing prevalence of inflammatory bowel disease and rising awareness about primary sclerosing cholangitis among people can boost demand for improved diagnostic and treatment options, thus, driving the market growth during the forecast period.
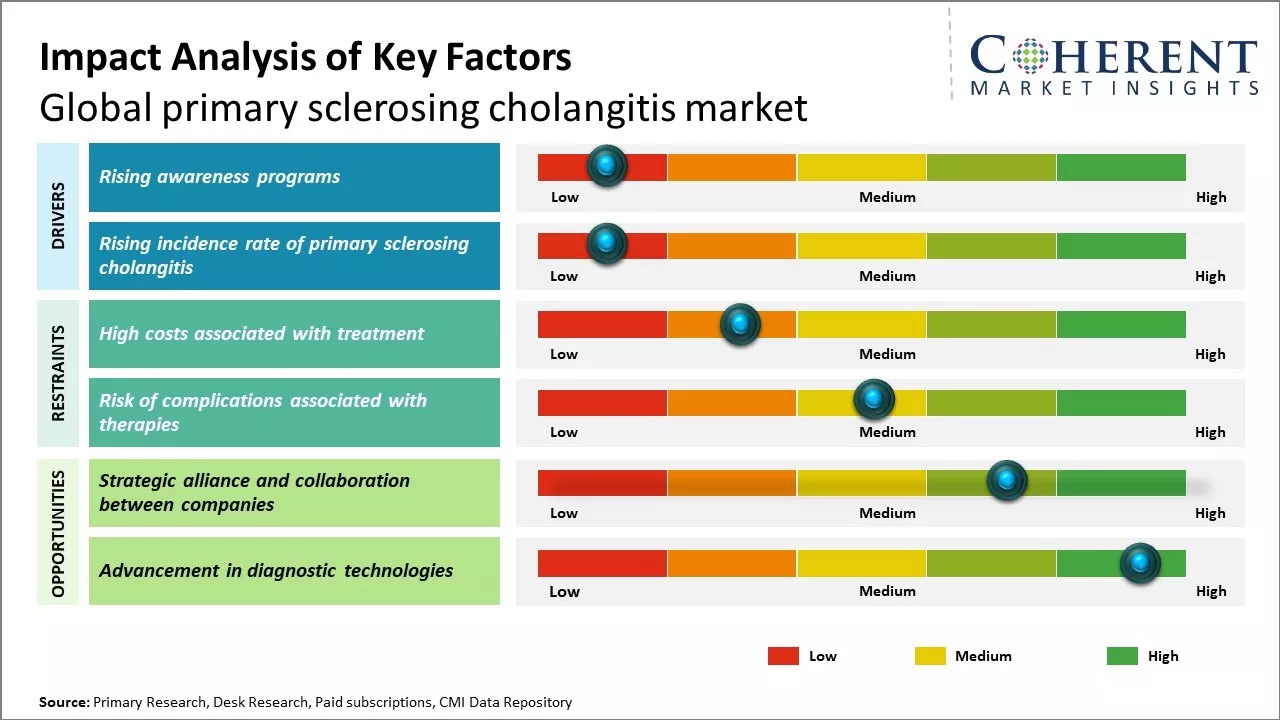
Rising incidence rate of primary sclerosing cholangitis
Rising incidence rate of primary sclerosing cholangitis can drive the market growth. Primary sclerosing cholangitis is a rare chronic disease that causes inflammation and scarring of the bile ducts. It occurs more commonly in men than women with a ratio of 2:1. The exact cause of primary sclerosing cholangitis is still unknown, however, genetics and autoimmunity play a major role. Primary sclerosing cholangitis often co-exists with inflammatory bowel disease particularly ulcerative colitis. Rising cases of ulcerative colitis across global populations, can increase the number of primary sclerosing cholangitis patients. Furthermore, better diagnosis and increasing awareness among physicians as well as patients have improved the rate of diagnosis over the past few decades. Earlier many cases would likely go undiagnosed due to non-specific symptoms in the initial stages. However now even mild cases are being identified which is contributing to the growth in incidence numbers. Key factors like improved healthcare infrastructure and emergence of sophisticated diagnostic techniques enables wide scale screening programs globally. This helps in early detection and diagnosis of the disease. Considering it is a chronic disease where patients require long term management, this rise in new patient cases can boost demand for primary sclerosing cholangitis drugs and therapies. For instance, in January 2023, according to a systematic review published by Clinical Gastroenterology and Hepatology Journal, rising incidence and prevalence of primary sclerosing cholangitis (PSC) across North America and Europe. The review, based on 17 population-based studies, found highest incidence rates in North America and Northern Europe, while prevalence was notably high in Finland and Minnesota. Researchers underscored the need for healthcare systems to adapt to manage this complex disease effectively, emphasizing the importance of early recognition, accurate diagnosis, and targeted care for PSC patients.
Get actionable strategies to beat competition: Download Free Sample
Rising awareness programs
Rising awareness programs plays a pivotal role in driving the growth of the global primary sclerosing cholangitis market. Various non-profit organizations like PSC Partners Seeking a Cure conduct awareness drives to educate people about the symptoms and management of this rare disease. Their educational initiatives like webinars, conferences and social media campaigns have increased understanding about primary sclerosing cholangitis (PSC) among the general public as well as healthcare community. This expanding awareness enables early detection of the condition. When diagnosed at an early stage, PSC can be managed better through lifestyle modifications and medical interventions. Awareness programs encourage people with symptoms to schedule an appointment with a gastroenterologist for timely diagnosis using techniques like magnetic resonance cholangiography. As more patients are diagnosed, there is huge demand for management and treatment options. Government bodies are also supporting awareness generation efforts. For instance, the National Organization for Rare Disorders (NORD), a non-profit organization funded by the U.S. Department of Health and Human Services, includes PSC in its rare disease database and provides educational materials on symptoms, testing and management. In 2021, according to NORD data, requests for PSC information grew by over 15% compared to 2020, reflecting the increasing awareness about this disease. In addition,, awareness campaigns are prompting research funding and development of novel drugs. Advocacy groups organize fundraisers to support clinical trials. As understanding increases about disease mechanisms, pathways and targets, pharmaceutical companies are spurred to develop effective therapies. For instance, in 2021, according to the study published by Clinics and Research in Hepatology and Gastroenterology, obeticholic acid was effective in improving biomarkers of PSC, indicating a treatment option.
Key Takeaways from Analyst:
Global primary sclerosing cholangitis market growth is driven by increasing prevalence of inflammatory bowel diseases. Rising research into novel treatment options can also drive the market growth. However, lack of approved drugs can hamper the market growth.
North America currently dominates the market due to strong research activities and growing cases of IBD. Europe is also a major revenue generator due to increasing government funding for rare disease research. However, Asia Pacific is likely to emerge as the fastest growing region.
By drug class, ursodeoxycholic acid is expected to dominate due to its first-line therapy status. Development of second-line treatments may threaten its long-term leadership potential. Novel biologics in clinical trials can address unmet needs in refractory cases. Successful pipeline candidates can transform the competitive scenario.
Reimbursement policies will also determine future market progress significantly. Investments into diagnostic technologies may improve early detection and widen treatable population. Physician education programs can boost awareness and diagnosis rate. However, high treatment costs poses challenge, especially in price-sensitive developing markets.
Market Challenges: High costs associated with treatment
The high costs associated with treatment can hamper the global primary sclerosing cholangitis market growth. Primary sclerosing cholangitis requires lifelong treatment and management as it is a chronic and progressive disease with no known cure. The treatment costs associated with PSC are immense as patients may require multiple medications, frequent doctor visits, diagnostic tests and some may eventually require liver transplantation. Liver transplantation is the only viable treatment option for patients with end stage liver disease due to PSC but it poses a huge financial burden. According to the data published by United Network for Organ Sharing in 2022, the average cost of liver transplantation in U.S. ranges between US$ 812,500– US$ 1,105,900. This includes costs related to pre-transplant care, surgery and post-transplant hospital stay and medications. Not only direct costs of the surgery but the lifelong immunosuppressive medication costs post-transplant can also add to the economic burden. In 2021, according to the report published by North American Transplant Coordinators Organization, lifetime drug costs post liver transplant is US$ 630,000 in the U.S. Most PSC patients require lifelong treatment with ursodeoxycholic acid, which is also very expensive. Many patients need repeated hospitalizations, abdominal surgeries like stricturoplasties or gastrointestinal stromal tumor resections costing tens of thousands each time. Lifelong management with diagnostic tests like MRCP or liver biopsies can also add to the costs.
Market Opportunities: Strategic alliance and collaboration between companies
Strategic alliances and collaborations between companies can offer growth opportunities for global primary sclerosing cholangitis market. PSC is a rare and serious liver disease with limited treatment options available. Joint efforts between pharmaceutical firms and research institutes could help expedite the development of novel and more effective therapies. When companies pool their complementary strengths, it allows for sharing of risks and costs associated with drug research and clinical trials. This enables smaller players to take on ambitious projects that would otherwise be beyond their individual capabilities. Larger corporations also benefit from the specialized expertise and technologies that partners bring to the table. These can accelerate progress across multiple promising research pathways simultaneously. International organizations like the PSC Partners Seeking a Cure are already facilitating collaborative research. These connect patients, clinicians and scientists across borders to advance understanding of this condition. Publicly accessible repositories of clinical data and biological samples are enabling more targeted research approaches. Transnational consortiums funded by the European Commission and foundations like the American Liver Foundation are actively pursuing projects on improved diagnostics, biomarkers and therapies.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
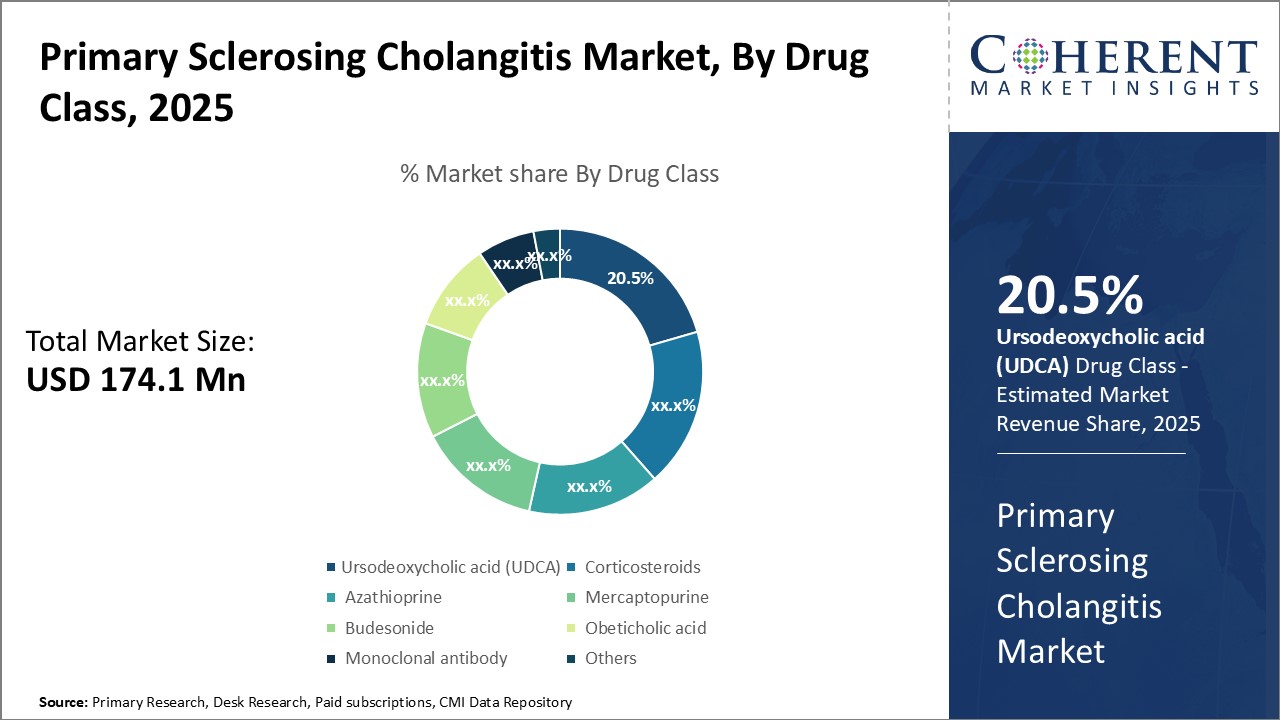
By Drug Class- Growing Patient Awareness Boosts UDCA Adoption
In terms of drug class, ursodeoxycholic acid (UDCA) segment is estimated to contribute the highest market share of 20.5% in 2025, owing to growing patient awareness of its effectiveness in managing primary sclerosing cholangitis (PSC). UDCA is known to improve liver test results and symptoms for many PSC patients, leading to reduced risks of complications like liver cancer. Its generic availability also makes it more affordable than newer treatment options. While other drugs play important roles, UDCA remains first-line therapy for PSC due to its well-established benefits and safety profile based on long-term use. Increasing diagnosis rates of PSC boosts demand for UDCA as patients and physicians prefer to choose a treatment with proven record of results. Moreover, engagement of patient advocacy groups in educating people about PSC and its management options helps grow recognition of UDCA.
By Route of Administration- Convenience of Oral Medication Boosts Segment Growth
In terms of route of administration, oral segment is estimated to contribute the highest market share of 80.5% in 2025, due to the convenience these offer to patients over alternative methods. For a chronic condition like PSC that has no cure, medication adherence becomes crucial to management. The non-invasive nature of oral drugs promotes compliance as patients find it less disruptive to their lifestyles versus therapies requiring hospital visits or intravenous administration. This is an important consideration for PSC given its unpredictable course that may involve lifelong treatment. With oral options, patients feel empowered to continuously monitor their condition without major lifestyle adjustments. The ease of use also allows elderly or busy patients to easily maintain treatment routines.
By Distribution Channel- Access through Hospitals Drives Segment
In terms of distribution channel, hospital pharmacies segment is estimated to contribute the highest market share of 40.5% in 2025, as these provide direct access to PSC treatments. As PSC involves periodic check-ups and management by gastroenterology specialists, hospitals serve as the primary points of diagnosis, treatment planning and medication access. This makes their pharmacies the preferred source for chronic PSC prescriptions. Hospital visits offer opportunities to address medication questions with clinicians, get financial aid and stay engaged in care - boosting long-term adherence important for conditions like PSC. Strong partnerships between hospitals and pharmaceutical companies also ensure adequate supplies of specialty drugs for PSC. With healthcare increasingly shifting to hospital-centered care models, their distribution channels will continue dominating for specialty conditions.
Need a Different Region or Segment? Download Free Sample
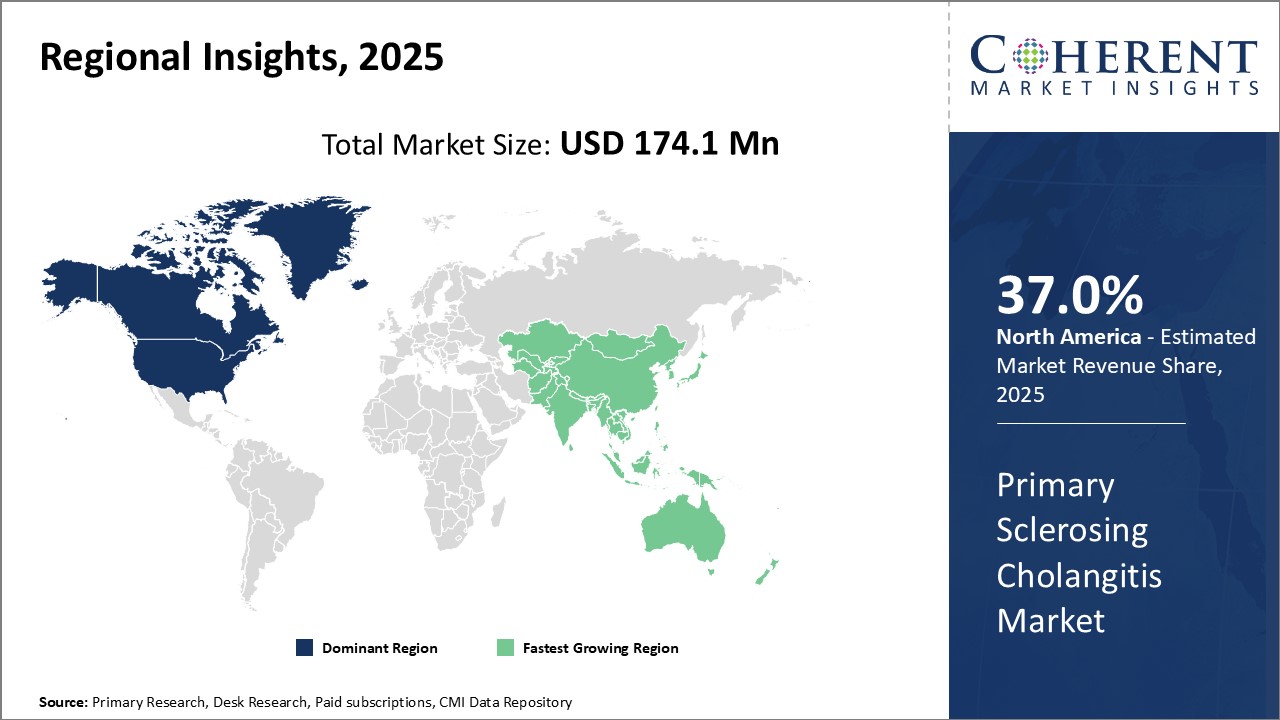
North America dominates the primary sclerosing cholangitis market with an estimated market share of 37.0% in 2025. The robust healthcare infrastructure and presence of most advanced treatments have enabled patients in the U.S. and Canada to gain early access to newer products. Hgh healthcare spending and availability of favorable reimbursement schemes boosts demand for high-priced drugs in this region. The region is also a major hub for pharmaceutical R&D activities. Leading pharmaceutical companies have their headquarters in the U.S. and invest heavily in clinical research and drug development for rare diseases like primary sclerosing cholangitis.
Asia Pacific market is poised to expand at the fastest rate during the forecast period. Rapid economic development, growing patient awareness about available therapies, and rising healthcare expenditure are driving the market growth in Asia Pacific. The significant patient population in China and India also works in favor of market players targeting these emerging countries. However, high treatment costs can limit the wider adoption of advanced treatments. Many local pharmaceutical companies have started developing low-cost biosimilars and generics to make therapies more affordable for a larger set of patients in Asia Pacific.
Primary sclerosing cholangitis Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 174.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.0% | 2032 Value Projection: | USD 298.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
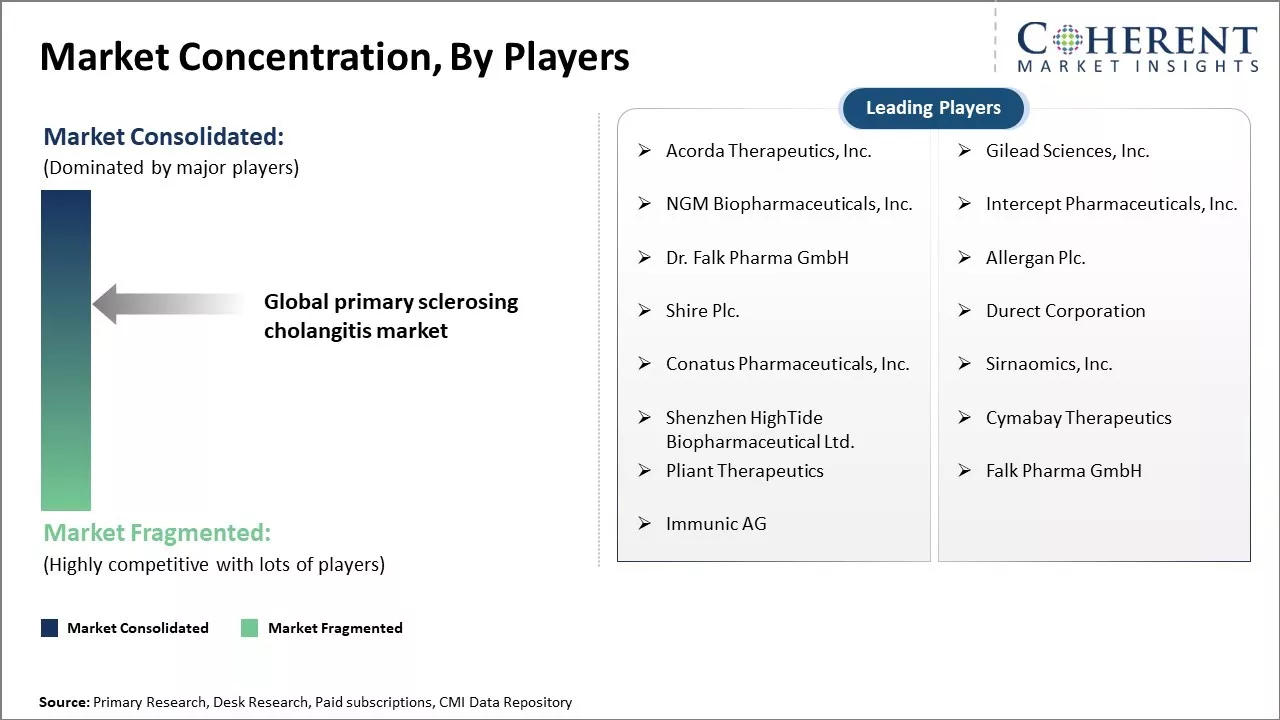
Acorda Therapeutics, Inc., Gilead Sciences, Inc., NGM Biopharmaceuticals, Inc., Intercept Pharmaceuticals, Inc., Dr. Falk Pharma GmbH, Allergan Plc., Shire Plc., Durect Corporation, Conatus Pharmaceuticals, Inc., Sirnaomics, Inc., Shenzhen HighTide Biopharmaceutical Ltd., Cymabay Therapeutics, Pliant Therapeutics, Immunic AG |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients