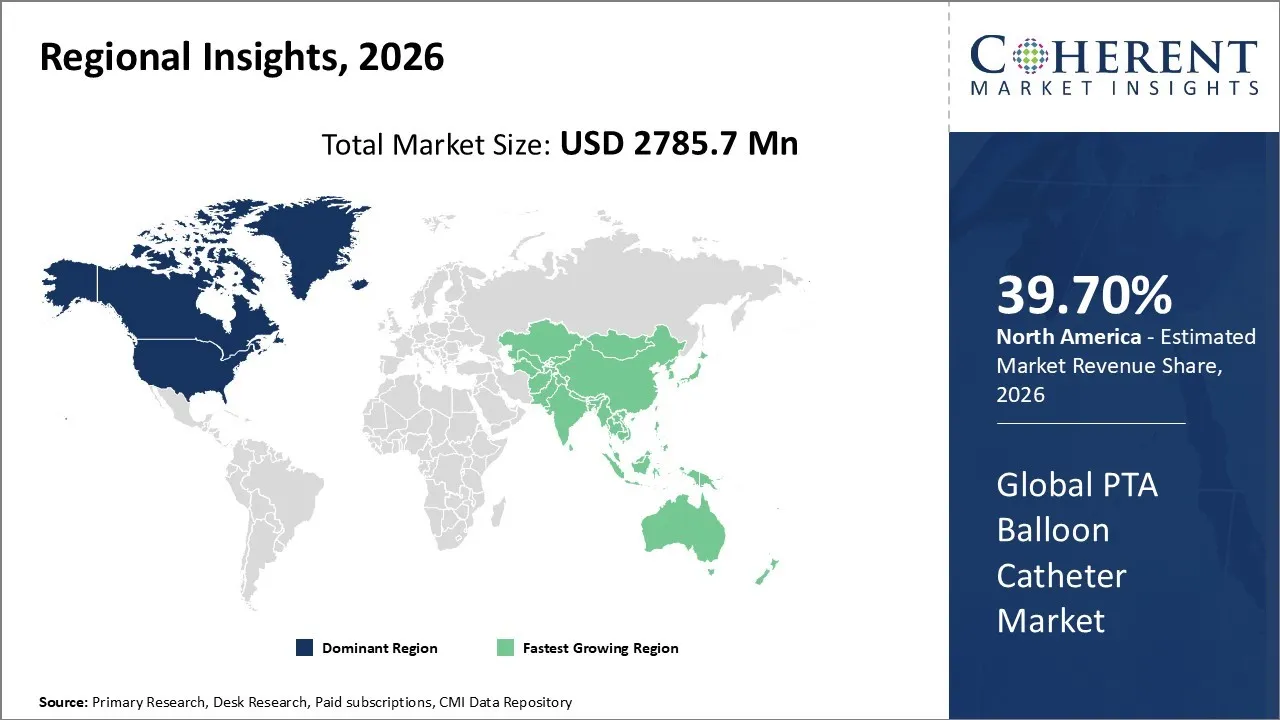
The PTA Balloon Catheter Market is estimated to be valued at USD 2,785.7 Mn in 2026 and is expected to reach USD 4,878.6 Mn by 2033, exhibiting a compound annual growth rate (CAGR) of 9.2% from 2026 to 2033.
Rising cases of peripheral artery disease and other cardiovascular disorders drive the PTA balloon catheter market, while clinicians increasingly prefer minimally invasive endovascular procedures. Manufacturers continue to advance balloon designs, introducing drug-coated and high-pressure variants that enhance treatment outcomes. Expanding geriatric populations, improving healthcare infrastructure, and broader access to interventional therapies further stimulate demand. Hospitals and ambulatory surgical centers actively perform most procedures, and North America leads the market by adopting innovative vascular technologies and maintaining highly advanced healthcare systems.
|
Current Events |
Description and its impact |
|
Geopolitical and Regulatory Developments |
|
|
Technological Advancements and Innovation |
|
|
Economic and Healthcare Infrastructure Factors |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Polyurethane holds the largest market share of 52.2% in 2026. Polyurethane strengthens the PTA balloon catheter market by delivering flexibility, durability, and strong biocompatibility that improve performance in complex vascular interventions. It withstands high-pressure inflation and maintains structural integrity, enhancing procedural safety and consistency. Manufacturers use polyurethane to design low-profile, highly trackable balloons that navigate tortuous vessels effectively. Continuous advancements in polymer engineering and improved compatibility with specialized coatings further accelerate its use, as healthcare providers prioritize efficient, reliable, and patient-centered solutions for minimally invasive angioplasty procedures. For instance, in January 2026, Covestro unveiled the CQ-Configurator, a digital tool that enables polyurethane value chain partners to quickly design and evaluate more sustainable foam solutions using Covestro’s environmental data.
Coronary Artery Disease expected to hold largest market share of 54.4% in 2026. The rising burden of coronary artery disease fuels demand in the PTA balloon catheter market as more patients undergo interventional procedures to reopen narrowed arteries and restore blood flow. Unhealthy lifestyles, including physical inactivity, smoking, diabetes, hypertension, and obesity, increase the occurrence of coronary blockages and drive the need for angioplasty. Healthcare providers leverage advanced diagnostic tools to detect disease earlier and expand cardiac care services, which accelerates procedural volumes and reinforces the adoption of balloon catheters in coronary interventions. For instance, in February 2025, Cagent Vascular unveiled Serranator SL-PRO percutaneous transluminal angioplasty (PTA) serration balloon catheter to treat chronic limb-threatening ischemia (CLTI) and pedal disease.
Hospitals acquired the prominent market share of 42.2% in 2026. Hospitals drive the PTA balloon catheter market by conducting large numbers of complex vascular procedures using advanced equipment and highly skilled medical teams. Their state-of-the-art catheterization labs, imaging technologies, and multidisciplinary expertise allow them to perform angioplasty safely and effectively. Hospitals handle emergency cases and referrals from smaller clinics, maintaining steady procedural demand. By leveraging favorable reimbursement policies and rapidly adopting new balloon catheter technologies, hospitals actively support the growth and widespread use of PTA balloon devices.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 39.70% in 2026. North America drives the PTA balloon catheter market by rapidly adopting advanced interventional technologies and leveraging robust healthcare infrastructure. Hospitals and specialized cardiac centers increasingly perform minimally invasive procedures for coronary and peripheral artery diseases, boosting procedural volumes. Well-established reimbursement systems, skilled clinicians, and widespread access to diagnostic and catheterization facilities support this growth. Manufacturers continually introduce innovative balloon designs, including drug-coated and high-pressure variants, while raising awareness of cardiovascular health further shapes market trends, solidifying North America’s role as a leading region for endovascular interventions. For instance, in February 2024, BIOTRONIK launched the Micro Rx catheter, a rapid-exchange microcatheter from IMDS, to improve guidewire support during percutaneous coronary interventions. This marks BIOTRONIK’s fourth IMDS product in the U.S., complementing its NHancer Rx, TrapIT, and ReCross catheter portfolio.
The Asia Pacific region drives growth in the PTA balloon catheter market as healthcare systems strengthen and access to advanced cardiovascular care improves. Rising cases of peripheral and coronary artery diseases, along with greater awareness of minimally invasive treatments, increase procedural demand. Hospitals and specialized cardiac centers actively adopt modern balloon catheter technologies, including drug-coated and high-pressure designs. Investments in medical infrastructure, training of skilled interventionalists, and supportive healthcare policies further accelerate balloon angioplasty procedures, establishing Asia Pacific as a rapidly expanding market for endovascular interventions.
The United States propels the PTA balloon catheter market by widely adopting advanced interventional technologies and leveraging a robust healthcare system. Hospitals and specialized cardiac centers perform minimally invasive procedures for coronary and peripheral artery diseases more frequently, increasing procedural volumes. Skilled clinicians, comprehensive diagnostic capabilities, and supportive reimbursement policies facilitate efficient treatment. Continuous innovation in balloon catheter designs, including drug-coated and high-pressure variants, along with rising patient awareness of cardiovascular health, actively drives market growth, establishing the United States as a key leader in endovascular interventions. For instance, Cardiovascular Systems announced the U.S. availability of the full OrbusNeich Jade percutaneous transluminal angioplasty (PTA) over-the-wire (OTW) balloon catheter line, serving as the exclusive distributor of OrbusNeich balloon products in the country.
India drives growth in the PTA balloon catheter market as increasing cardiovascular disease cases and greater awareness of minimally invasive treatments boost demand for angioplasty procedures. Hospitals and specialized cardiac centers actively adopt advanced balloon catheter technologies, including drug-coated and high-pressure designs, to enhance patient outcomes. Investments in healthcare infrastructure, training skilled interventional cardiologists, and government initiatives supporting cardiovascular care expand procedural capacity. Along with improved access to diagnostic and treatment facilities, these efforts establish India as a rapidly emerging and dynamic market for endovascular interventions.
Healthcare providers increasingly favor minimally invasive procedures over traditional surgeries for coronary and peripheral artery diseases. PTA balloon catheters enable faster recovery, reduced hospital stays, and lower complication rates. This trend is driving innovation in catheter design, focusing on low-profile, flexible, and trackable balloons that enhance procedural efficiency and patient comfort during complex angioplasty procedures.
Manufacturers are developing drug-coated, high-pressure, and specialty balloons to improve procedural outcomes and reduce restenosis. Innovations include scoring and cutting balloons for complex lesions, and enhanced materials like polyurethane for durability and flexibility. These technological advancements respond to physician demands for safer, more effective devices suitable for diverse vascular anatomies and challenging interventions.
Manufacturers have the opportunity to expand in advanced balloon designs, including drug-coated, scoring, and high-pressure variants. These innovations reduce restenosis and improve procedural success, especially in complex coronary and peripheral artery lesions. By addressing unmet clinical needs, companies can differentiate products, enhance physician adoption, and increase market penetration across hospitals and specialty cardiac centers globally.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 2,785.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.2% | 2033 Value Projection: | USD 4,878.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Terumo, Cardinal Health, Boston Scientific, AndraTec, Cook Medical, Biotronik, Abbott, Creagh Medical, TriReme Medical, Natec Medical, Surmodics, Inc, B. Braun Melsungen AG, Becton Dickinson and Co, and Acotec Scientific Co Ltd |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients