Small Molecule Prefilled Syringes Market is estimated to be valued at USD 21.01 Bn in 2025 and is expected to reach USD 29.96 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.2% from 2025 to 2032.
Analysts’ Views on Global Small Molecule Prefilled Syringes Market:
A pre-filled syringe is a disposable syringe that is already filled with the substance to be injected. The prefilled syringe that already contains the injection liquid. A prefilled syringe is a single dose of medication with a needle attached by the manufacturer. Increasing new product launch by market players, increasing number of chronic diseases, adoption of self injection devices are expected to create lucrative growth opportunities in the market.
Figure 1. Global Small Molecule Prefilled Syringes Market Share (%), By Type, 2025
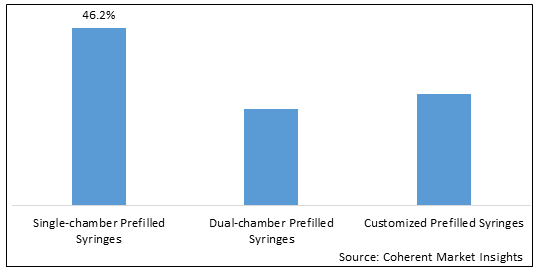
To learn more about this report, Download Free Sample
Global Small Molecule Prefilled Syringes Market– Drivers
Figure 2. Global Small Molecule Prefilled Syringes Market Share(%), By Region, 2025
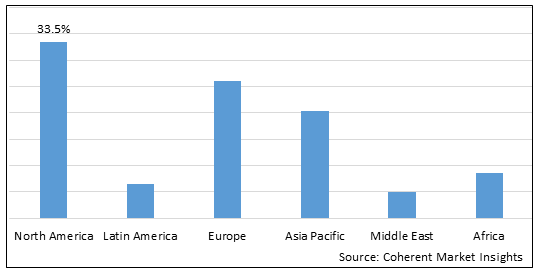
To learn more about this report, Download Free Sample
Global Small Molecule Prefilled Syringes Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global small molecule prefilled syringes market over the forecast period. North America is estimated to hold 33.5 % of the market share in 2025. North America small molecule prefilled syringes market is expected to witness significant growth in thenear future due toincreasing product approvals by the regulatory authorities. For instance, in February 2022, Takeda Pharmaceutical Company Limited, a pharmaceutical company, announced that it had received approval by the U.S. Food and Drug Administration (FDA) for its single-dose prefilled injection syringe (PFS) named TAKHZYRO (lanadelumab-flyo) for the prevention of attacks of hereditary angioedema (HAE) in adult and pediatric patients of age 12 years and older. The PFS is ready-to-use and requires fewer preparation steps than the current TAKHZYRO vial injection, while reducing supplies and waste.
Global Small Molecule Prefilled Syringes Market– Impact of Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic and lockdown in various countries across the globe has impacted the financial status of businesses across all sectors including private healthcare sector. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions. The COVID-19 pandemic has affected the economy of various regions across the globe in three main ways; 1) by directly affecting the production and demand; 2) by creating disruptions in distribution channels; and 3) through its financial impact on companies and financial markets. Several countries such as Thailand, Indonesia, and Singapore faced problemsproblems regardingtransportation and distribution of healthcare products.
In 2020, the COVID-19 pandemic disrupted health-care delivery around the world including delay in needle or syringe related diagnosis and treatment. Therefore, the impact of COVID-19 is expected to hamper growth of global small molecule prefilled syringes market. For instance, in September 2020, according to an article published by International Journal of Drug Policy, the needle and syringe programs (NSPs) are organized for population for free access to injectables and for awareness of the adverse effects associated with injecting drugs. According to the same source, the restrictions due to the pandemic resulted in a 36% decrease in the number of needle and service programs (NSP) clients in England, a 36% decrease in visits, and a 29% decrease in needle distribution as compared to 2019. NSP coverage for those injecting psychoactive drugs was cut in half, falling from 14 needles per week on March 15, 2020, to seven needles per week by mid-April 2020.
Small Molecule Prefilled Syringes Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 21.01 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.2% | 2032 Value Projection: | USD 29.96 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
BD, Cytiva, Merck KGaA, Gaplast, Ascendia Pharmaceuticals, Sanofi, Viatris Inc. (Mylan N.V.), Pfizer Inc., Dr. Reddy’s Laboratories Ltd., Fresenius Kabi AG and McKesson Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Small Molecule Prefilled Syringes Market Segmentation:
Global small molecule prefilled syringes market is segmented into type, material type, application, distribution channel, and region.
Global Small Molecule Prefilled Syringes Market- Cross Sectional Analysis
Key players operating in the global small molecule prefilled syringes market are using advanced technology in emerging economies, and this is expected to drive small molecule prefilled syringes market in Asia Pacific region. For instance, in March 2020, Xeris Pharmaceuticals, Inc., a pharmaceutical company, announced that it is developing of using theirnovel formulation technology platforms to develop and commercialize ready-to-use injectable and infusion drug formulations emphasizes the importance of Gvoke PFS for people with diabetes and encourages healthcare professionals to talk to their at-risk patients about the availability of glucagon.
Global Small Molecule Prefilled Syringes Market: Key Developments
Global Small Molecule Prefilled Syringes Market: Key Trends
Introduction of small molecule prefilled syringes with advanced technology: Introduction of small molecule prefilled Ssyringes with advanced technology in the market is expected to drive growth of the global small molecule prefilled syringes market over the forecast period. For instance, in September 2022, BD, a global medical technology company, introduced a next-generation prefillable glass syringe (PFS) that sets a new performance standard for vaccine PFS with new and tightened processability specifications : cosmetics, contaminants and integrity.
Global Small Molecule Prefilled Syringes Market: Restraint
Global Small Molecule Prefilled Syringes Market - Key Players
Major players operating in the global small molecule prefilled syringes market include BD, Cytiva, Merck KGaA, Gaplast, Ascendia Pharmaceuticals, Sanofi, Viatris Inc. (Mylan N.V.), Pfizer Inc., Dr. Reddy’s Laboratories Ltd., Fresenius Kabi AG and McKesson Corporation.
Definition: A prefilled syringe (PFS) is a needle-based injection method that has been pre-filled with the substance that will be delivered. For drug containment and delivery, many autoinjectors rely on a prefilled glass syringe. This prefilled syringe is made up of several components that must be verified using a range of standard tests in order to establish the integrity and operation of the syringe system and, ultimately, ensure proper drug containment and expulsion and the highest level of comfort for the patient.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients