Techniques used to analyse biological markers in genome and proteome are the collection of molecular diagnostics. To monitor and diagnose the disease, detect risk and to make the decision regarding the therapies for individual patient, this technique is used. The necessary underpinnings are supplied by molecular diagnostics for any successful implementation of gene therapy or biologic response modifiers. For detecting minimal residual disease, therapy responses, and assessing disease prognosis the molecular diagnostics offers a great tool.
Moreover, laboratories are having greater impact by adopting molecular diagnostics, on care and treatment of patients. These modern systems are easing doctors to treat infectious diseases fast and with much accuracy.
Figure 1. U.S., Europe, and Asia Pacific Molecular Diagnostics Market (US$ Mn), By Product Type, 2021
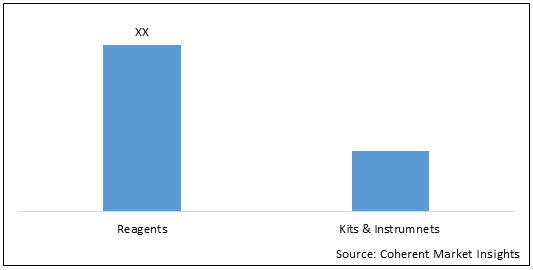
To learn more about this report, Download Free Sample
Figure 1.1. U.S., Europe, and Asia Pacific Molecular Diagnostics Market (US$ Mn), By Product Type, 2028
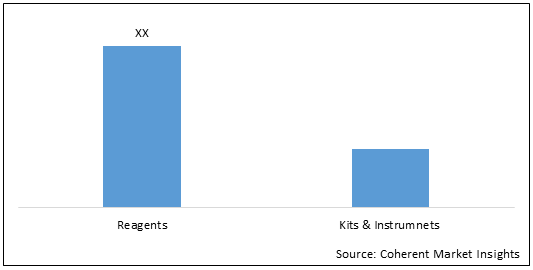
To learn more about this report, Download Free Sample
U.S., Europe, and Asia Pacific Molecular Diagnostics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 7,147 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 10.7% | 2028 Value Projection: | US$ 11,000 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, Hologic, Inc., Grifols, S.A., Qiagen N.V., F.Hoffmann-La Roche Ltd., Siemens Healthineers, Becton, Dickinson and Company, Beckmann Coulter, Inc., Bio-Rad Laboratories, and Thermo Fisher Scientific Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. U.S., Europe, and Asia Pacific Molecular Diagnostics Market (US$ Mn), By Technology, 2021
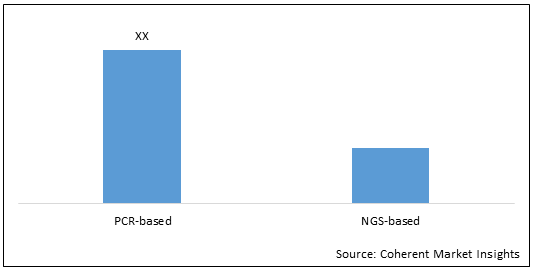
To learn more about this report, Download Free Sample
Figure 2.1. U.S., Europe, and Asia Pacific Molecular Diagnostics Market (US$ Mn), By Technology, 2028
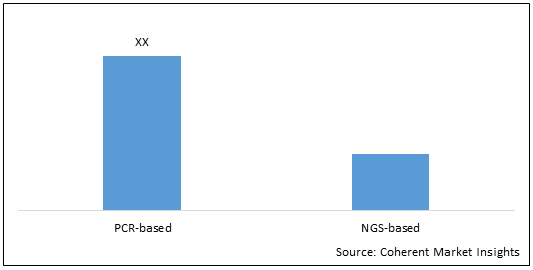
To learn more about this report, Download Free Sample
U.S., Europe, and Asia Pacific Molecular Diagnostics Market – Impact of Coronavirus (COVID-19) Pandemic
The coronavirus (COVID-19) pandemic is the most recent outbreak, which was first reported on December 31, 2019, in Wuhan, China.
The COVID-19 has impacted molecular diagnostics testing and research for non-COVID applications. The deployment of COVID-19 molecular diagnostics slowed down due to lack of equipment, insufficient lab environmental protection, and reagent capacity. Even the laboratory professionals faced immediate urgent testing demands. On non-COVID biologic research, a less adverse consequence has been noticed which also includes molecular diagnostics. By reallocation and equipment, the non-COVID research has been hampered a lot since past year.
Furthermore, among the sectors, hospital based clinical research were most severely affected.
U.S., Europe, and Asia Pacific Molecular Diagnostics Market Restraint
High cost of instruments and regulatory framework in developed economies (Canada and most of Western Europe) are hampering growth of the U.S., Europe, and Asia Pacific Molecular Diagnostics Market.
According to National Library of Medicine, the regulatory landscape struggles to manage the concerns for molecular diagnostics, by treating diagnostics on primary base whether the kit is sold or offered, rather than based on risk. According to National Library of Medicine, in the U.S., the regulatory system for diagnostics is problematic, with inconsistent regulatory pathways and weakness.
Key Players
Major players operating in the U.S., Europe, and Asia Pacific Molecular Diagnostics Market include Abbott Laboratories, Hologic, Inc., Grifols, S.A., Qiagen N.V., F.Hoffmann-La Roche Ltd., Siemens Healthineers, Becton, Dickinson and Company, Beckmann Coulter, Inc., Bio-Rad Laboratories, and Thermo Fisher Scientific Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients