Intravenous immunoglobulin (IVIG) is a therapy treatment for patients who are immunocompromised. IVIG is a collection of immunoglobulins (antibodies) derived from the plasma of thousands of healthy donors.
Immunoglobulins are proteins produced by the immune system of healthy people to aid in the fight against infections. While IVIG is derived from plasma (a blood product), it is so pure that the risk of contracting a blood-borne infection is extremely low.
IVIG is administered as an intravenous infusion (via a vein in the arm) that takes several hours to complete. The frequency of infusions varies depending on the patient's needs.
U.S. Intravenous Immunoglobulin Market - Impact of the Coronavirus (COVID-19) Pandemic
Coronavirus (COVID-19) outbreak was first reported on December 31, 2019, in Wuhan, China. The World Health Organization declared COVID-19 as pandemic on March 11, 2020. According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization, over 234 million cases and 4.80 million deaths due to coronavirus (COVID-19) were reported till October 04, 2021, across the globe.
Impact of COVID-19 on Demand and Supply of Intravenous Immunoglobulin
The COVID-19 pandemic and lockdown in various countries across the globe have impacted the financial status of businesses across all sectors including private healthcare sector. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions. The COVID-19 pandemic has affected the economy of various regions across the globe in three main ways; 1) by directly affecting the production and demand; 2) by creating disruptions in distribution channels; and 3) through its financial impact on companies and financial markets. Several countries such as Thailand, Indonesia, and Singapore are facing problems with regards to transportation and distribution of healthcare products.
However, impact of the coronavirus (COVID-19) pandemic is expected to drive the growth of the U.S. intravenous immunoglobulin market owing to increasing research and development activities of intravenous immunoglobulins (IVIG) for the treatment of COVID-19. For instance, according to the article published in Respiration journal, in January 2021, early IVIG administration was associated with reduced ventilator use, hospital and intensive care unit length of stay in a retrospective study conducted in the U.S. and Germany including patients from three hospitals.
The U.S. intravenous immunoglobulin market is estimated to be valued at US$ 6.33 Bn in 2021, and is expected to exhibit a CAGR of 6.8% over the forecast period (2021-2028).
U.S. Intravenous Immunoglobulin Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 6.33 Bn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 6.8% | 2028 Value Projection: | US$ 10.06 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Biotest AG, Octapharma AG, Grifols S.A., Kedrion Biopharma, Inc., CSL Behring, McKesson Corporation, Takeda Pharmaceutical Company Limited, Bio Products Laboratory Ltd., Pfizer, Inc., and ADMA Biologics, Inc. |
||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 1: U.S. Intravenous Immunoglobulin Market Share (%) Analysis, By Formulation, 2021
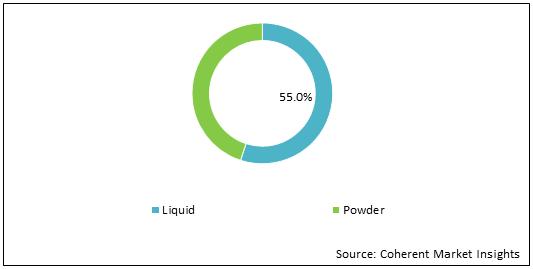
To learn more about this report, Download Free Sample
The increasing cases of Kawasaki disease for which intravenous immunoglobulins are used as a treatment, is expected to drive growth of the market over the forecast period.
The emerging prevalence of Kawasaki disease is expected to bolster the growth of the U.S. intravenous immunoglobulin market. Kawasaki disease (KD), also known as Kawasaki syndrome, is an unknown etiology acute febrile illness that primarily affects children under the age of five. For instance, according to the Centers for Disease Control and Prevention (CDC), in 2016, about 5440 hospitalizations for KD were reported among children under the age of 18 in the U.S.; 3935 of these children were under the age of five, resulting in a hospitalization rate of 19.8 per 100,000 children in that age group.
Approvals from Regulatory Authorities
Increasing product approvals from the regulatory authorities for the intravenous immunoglobulins is expected to provide lucrative growth opportunities for players in the U.S. intravenous immunoglobulin market. For instance, on May 20, 2020, the U.S. Food and Drug Administration (FDA) approved an investigational new drug (IND) application submitted by Octapharma U.S., one of the largest human protein product manufacturers, for a phase three clinical trial on the efficacy and safety of Octagam 10 percent [Immune Globulin Intravenous (Human)] therapy in COVID-19 patients with severe disease progression.
Figure 2: U.S. Intravenous Immunoglobulin Market Share (%) Analysis, By Application, 2021
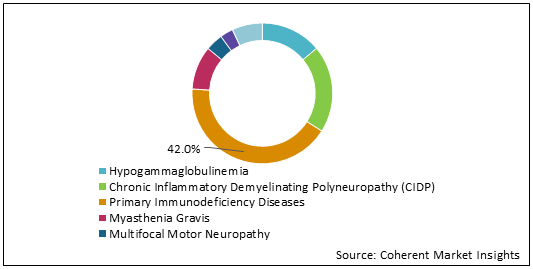
To learn more about this report, Download Free Sample
U.S. Intravenous Immunoglobulin Market – Competitive Landscape
Major players operating in the U.S. intravenous immunoglobulin market include Biotest AG, Octapharma AG, Grifols S.A., Kedrion Biopharma, Inc., CSL Behring, McKesson Corporation, Takeda Pharmaceutical Company Limited, Bio Products Laboratory Ltd., Pfizer, Inc., and ADMA Biologics, Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients