U.S. Uterine Fibroid Treatment Market is estimated to be valued at USD 291.0 Mn in 2025 and is expected to reach USD 382.9 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4% from 2025 to 2032.
Analysts’ Views on U.S Uterine Fibroids Treatment Market:
Over the forecast period, the U.S. uterine fibroids treatment market is anticipated to rise because of the high prevalence of uterine fibroids among women, the adoption of minimally invasive therapies, and robotic procedures. For instance, in February 2021, according to the Office of the Assistant Secretary for Health (OASH) at the U.S. Department of Health and Human Services, about 20-80 % of women develop fibroids by the time they reach age 50, and fibroids become more common as women age, especially during the 30s and 40s through menopause.
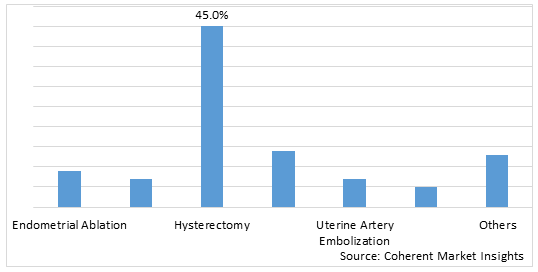
Figure 1. U.S Uterine Fibroids Treatment Market Share (%), By Procedure, 2025
To learn more about this report, Download Free Sample
U.S Uterine Fibroids Treatment Market–Drivers
High incidence of uterine fibroids among women
Increasing incidence of uterine fibroids among women is expected to boost growth of the U.S uterine fibroids treatment market over the forecast period. For instance, on May 13 2023, according to an article published in the Journal of Biomed Central Women's Health, the prevalence of UL (Uterine leiomyomata (Fibroids)) was approximately 12.92% in U.S. in 2020, and is estimated to increase up to 80 % by 2025.
Rising number of hysterectomy
The rising number of hysterectomy surgical procedures is one of the main drivers of market growth. Furthermore, this method is the most popular and well-proven permanent treatment for uterine fibroids. As a result, the hysterectomy will likely grow as the number of related surgeries increases. For instance, in November 2020, according to an article published in the Journal of Gynecologic Oncology, according to the Centers for Disease Control and Prevention (CDC), about 600,000 hysterectomies are performed in the United State each year. For instance in November 2022, according to the American College of Obstetricians and Gynecologists, around a third of all women will have a hysterectomy by age 60.
U.S Uterine Fibroids Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the U.S uterine fibroids treatment market. For instance, on July 6, 2021, according to an article published in the Journal of PLOS ONE, a scientific journal for science and medicine, discussed the impact of the COVID-19 pandemic on waiting times for elective surgery patients. Where the study concluded that the health care service providers patients will have increased elective surgery waiting times by one-third due to the pandemic, however the elective surgery rate will increase by one-fifth after the pandemic. This shows a drop in the usage of laparoscopic power morcellators, which could have a negative impact on the U.S uterine fibroids treatment market growth.
U.S. Uterine Fibroids Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 291.0 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4% | 2032 Value Projection: | USD 382.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Blue Endo, Boston Scientific Corporation or its affiliates, CooperSurgical, Inc., Karl Storz SE & Co. KG, Myovant Sciences GmbH (Sumitovant Biopharma Ltd.), Halt Medical, Inc. (Hologic, Inc.), LiNA Medical ApS, Merit Medical Systems, Olympus Corporation, and Richard Wolf GmbH. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S Uterine Fibroids Treatment Market- Segmentation:
The U.S. uterine fibroids treatment market is segmented into procedure type, and end user.
Based on Procedure Type, the U.S. uterine fibroids treatment market is segmented into Endometrial Ablation, MRI Guided Procedures (MRI-guided Percutaneous Laser Ablation, and MRI-guided Transcutaneous Focused Ultrasound), Hysterectomy (Abdominal Hysterectomy, Vaginal Hysterectomy, Laparoscopic Hysterectomy, Robotic Hysterectomy, and Hysteroscopic Morcellation), Myomectomy (Open Myomectomy, Laparoscopic Myomectomy, and Robotic Myomectomy), Uterine Artery Embolization, Radiofrequency Ablation, and Others. Out of which, the hysterectomy segment is expected to dominate the U.S. uterine fibroids treatment market during the forecast period and this is due to the increase in the hysterectomies.
Based on End user, the U.S. uterine fibroids treatment market is segmented into Hospitals and Ambulatory Surgical Centers. Out of which, the hospitals segment is expected to dominate the market over the forecast period and this is attributed to the increasing prevalence of uterine fibroids.
Among all the segmentations, the End User segment has the highest potential due to the increasing prevalence of uterine fibroids which leads to the increase in hospitalizations for the treatment over the forecast period. For instance, in February 2022, according to an article published by contemporary obstetrician-gynecologist (OB/GYN) estimated that over 70% of women are estimated to develop uterine fibroids by age 50 in U.S.
U.S Uterine Fibroids Treatment Market: Key Developments
Major players in the market are focused on R&D to expand their product portfolio. For instance, in April 2020, Sumitovant Biopharma Ltd., a technology-driven biopharma announced publication of abstracts from Myovant Sciences regarding the efficacy and safety of relugolix combination therapy in women with heavy menstrual bleeding associated with uterine fibroids, in the journal Obstetrics & Gynecology.
In June 2021, Hologic, Inc., a medical technology company, primarily focused on women’s health; it sells medical devices for diagnostics, surgery, and medical imaging, announced that Cigna, a multinational managed healthcare and insurance company, has updated its medical policy to cover the Acessa Laparoscopic Radiofrequency Ablation (Lap-RFA) procedure (CPT code 58674) as medically necessary.
In January 2022, Hologic, Inc. a medical technology company, primarily focused on women’s health; it sells medical devices for diagnostics, surgery, and medical imaging announced, the launch of Hey, U!, an educational campaign focused on uterine health due to a lack of awareness among U.S. women about uterine health, including uterine fibroid symptoms and treatment options.
In November 2022, Olympus Corporation, a global technology in designing and delivering innovative solutions for medical and surgical procedures, announced the market launch of the moresolution Power Morcellator, which is manufactured by TROKAMED GmbH and is available in the U.S. through a distribution agreement with Olympus America, Inc. The moresolution Morcellator is designed for advanced gynecologic procedures with large, calcified tissue specimens.
U.S Uterine Fibroids Treatment Market: Key Trends
Investigating novel hormonal medical therapy
Increasing need to treat heavy menstrual bleeding and surging demand of novel hormonal medical therapy, the U.S uterine fibroids treatment market is expected to experience considerable expansion over the forecast period. For instance, in March 2022, according to an article published in the Journal of Yangtze Medicine, Ulipristal acetate (UPA) has been the first selective progesterone-receptor modulator (SPRM) approved for the pre-operative and long-term management of uterine fibroids. The use of ulipristal acetate is supported by research suggesting that progesterone pathways play a significant role in the pathogenesis of uterine fibroids. Alternatives to surgical intervention must be available, especially for people who want to maintain their fertility and uterus. Ulipristal acetate is one of the alternatives that has been shown to be useful in treating fibroid symptoms.
U.S Uterine Fibroids Treatment Market: Restraint
Complications associated with the morcellators
Complications associated with morcellators are expected to hinder growth of the U.S. uterine fibroids treatment market. Laparoscopic power morcellators are used in hysterectomy and myomectomy procedures for the treatment of uterine fibroids. However, power morcellators could cause complications such as metastatic leiomyosarcoma cancer. So, avoiding the use of morcellators, and the use of other laparoscopic techniques to be followed for uterine fibroid removal. For instance, in November 2022, according to an article published by the Drugwatch, surgery with a morcellator has higher risks such as Intra-abdominal abscesses, Organ damage, Bleeding or infection and many others. Even non-cancerous fibroid tissue may be spread by a morcellator. Non-cancerous fibroid tissue can attach to other tissues and organs in the abdominal cavity, causing more fibroids to grow.
U.S Uterine Fibroids Treatment Market - Key Players
Major players operating in the U.S. uterine fibroids treatment market include Blue Endo, Boston Scientific Corporation or its affiliates, CooperSurgical, Inc., Karl Storz SE & Co. KG, Myovant Sciences GmbH (Sumitovant Biopharma Ltd.), Halt Medical, Inc. (Hologic, Inc.), LiNA Medical ApS, Merit Medical Systems, Olympus Corporation, and Richard Wolf GmbH.
Definition: Uterine fibroids, also known as uterine leiomyoma’s, are the most common tumors affecting the female reproductive tract. These tumors disrupt the function of uterus and causes anemia, excess bleeding, and pelvic discomfort. There are various treatment options available for the treatment of the condition such as drug therapy and surgical procedure. These are the most common tumor of the female reproductive tract. Though they are non-cancerous, they can seriously injure adjacent organs and result in anemia from excessive menstruation. Uterine fibroids are benign tumors made of muscular and fibrous tissues, typically developing in or on the walls of the uterus.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients