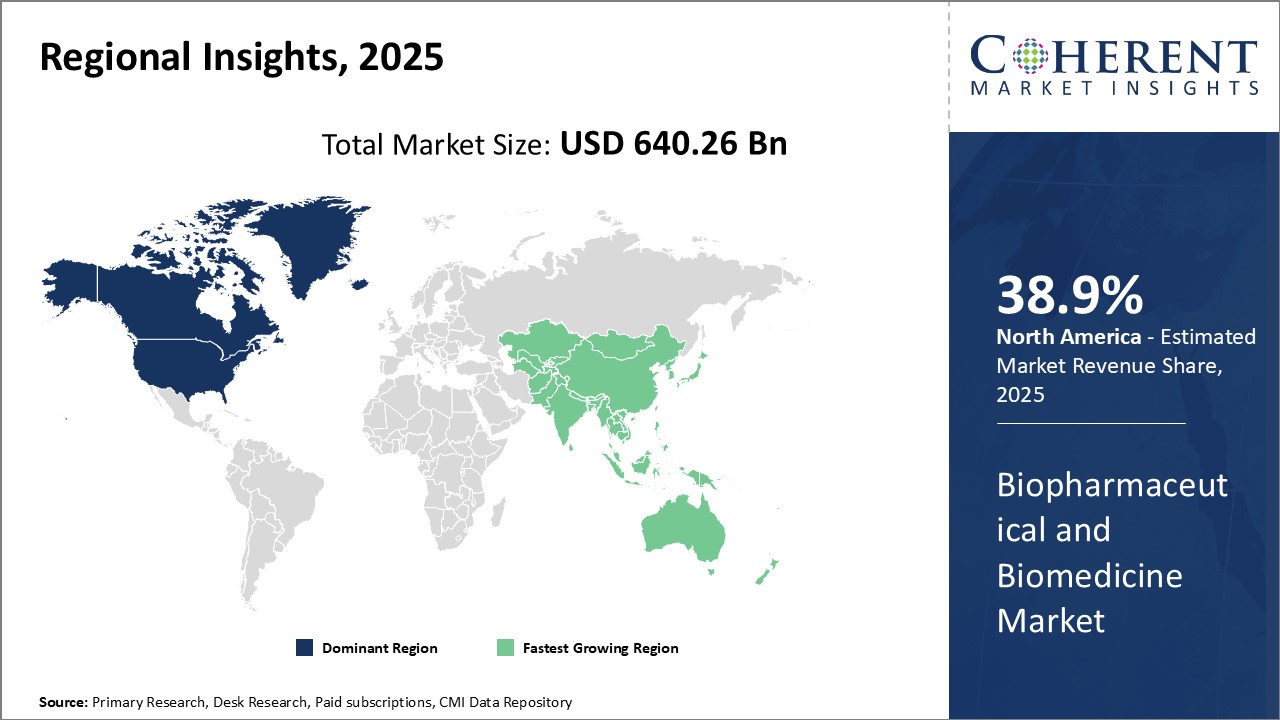
The Global Biopharmaceutical and Biomedicine Market is estimated to be valued at USD 640.26 Bn in 2025 and is expected to reach USD 1,425.17 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.1% from 2025 to 2032.
Key Takeaways of the Biopharmaceutical and Biomedicine Market:
Market Overview:
Advancements in biomedical research have fueled the development of innovative therapeutic drugs and diagnostics. Significant investments by industry players and government organizations in R&D of novel biologics, cell, and gene therapies for the treatment of chronic diseases such as cancer and diabetes are also supporting market growth. The market is witnessing increasing adoption of personalized medicine aided by advancements in precision medicine and availability of patient specific data. Integration of AI and machine learning tools in drug discovery is also emerging as a key trend. Biopharmaceutical companies are exploring opportunities in developing biotherapeutics targeting disorders with high unmet needs.
Product Type Insights - Focus on core therapeutic areas drives growth of biopharmaceuticals
In terms of product type, the biopharmaceuticals segment is expected to contribute the highest share of the market with 40.6% in 2025 owing to increased focus on developing novel biologics for core therapeutic areas. Biopharmaceuticals include recombinant proteins, monoclonal antibodies, vaccines, blood factors, cell and gene therapies which have revolutionized the treatment of various life-threatening diseases. Large pharmaceutical companies are actively pursuing both in-house development and strategic collaborations to strengthen their biologics pipeline. Successful product approvals and launches in areas of oncology, immunology and neurodegenerative diseases have boosted sales volumes.
Application Insights - Therapeutics dominates driven by aging population and rise of chronic diseases
In terms of application, the therapeutics segment is expected to contribute the highest share of the market with 45.6% in 2025 driven by the growing prevalence of chronic conditions worldwide coupled with an aging population. Diseases like cancer, diabetes, and cardiovascular disease pose a huge burden and therapeutic treatments are in higher demand. Biopharmaceutical innovations have massively expanded treatment options over the last two decades with advanced biologics, targeted therapies, and combination regimens.
Type Insights - Branded Biopharmaceuticals lead over affordability concerns of generics
In terms of type, the branded biopharmaceuticals segment is expected to contribute the highest share of the market with 52.8% in 2025 owing to perceived advantages over generics in safety, efficacy, and data exclusivity. Biologics are complex molecules manufactured through living systems and face formidable scientific hurdles for exact generic replication. Unlike conventional drugs, proven quality assurance of generic copies has not been well established. Pharmacovigilance also becomes critical for branded versions which have accumulated long-term clinical data on performance and adverse events.
Need a Different Region or Segment? Download Free Sample
North America Biopharmaceutical and Biomedicine Market Trends
In North America, the dominance in the biopharmaceutical and biomedicine market with a share of 38.9% in 2025 can be attributed to robust research and development funding, presence of leading global pharmaceutical companies, and favorable regulatory environment conducive for drug innovation. The region also attracts significant investment and business opportunities due to its highly skilled workforce and sophisticated healthcare infrastructure.
Asia Pacific Biopharmaceutical and Biomedicine Market Trends
Meanwhile, the Asia Pacific region exhibits the fastest growth and emerging as an important hub with share of 29.2% in 2025. Major factors driving the growth include rising healthcare expenditure, growing middle-class demographics seeking higher quality care, and government initiatives encouraging local manufacturing and international partnerships. Leading players such as GSK Plc are particularly leveraging opportunities in China, India, and Southeast Asian countries.
Biopharmaceutical and Biomedicine Market Outlook for Key Countries
United States Biopharmaceutical and Biomedicine Market Trends
The U.S. leads the biopharmaceutical sector, with substantial investments in research and development. In December 2022, members of the Pharmaceutical Research and Manufacturers of America (PhRMA) invested approximately US$ 101 billion in R&D, underscoring the country's commitment to innovation.
Germany Biopharmaceutical and Biomedicine Market Trends
Germany is a leader in Europe for biopharmaceuticals, supported by strong regulatory frameworks and funding for research. In October 2024, the German government announced a US$ 1.04 billion investment in biotechnology research, aimed at enhancing the development of innovative therapies. This initiative is likely to accelerate advancements in the sector.
China Biopharmaceutical and Biomedicine Market Trends
Th China biopharmaceutical market is expanding rapidly, attracting global pharmaceutical companies seeking acquisitions to enhance their drug pipelines. In February 2024, AstraZeneca acquired Gracell Biotechnologies for US$ 1.2 billion, reflecting the strategic interest in China's innovative biotech firms.
Japan Biopharmaceutical and Biomedicine Market Trends
Japan’s expanding capabilities in biopharmaceutical manufacturing and its focus on cutting-edge therapies position it as a global leader in the industry. AGC Inc., a world-leading manufacturer of glass, is investing approximately 50 billion to expand its biopharmaceutical contract development and manufacturing organization (CDMO) capabilities at its Yokohama Technical Center in Japan. The expansion will support the development of gene and cell therapies starting in 2025, and manufacturing services for mRNA pharmaceuticals, biopharmaceuticals using mammalian cell cultures by 2026.
Get actionable strategies to beat competition: Download Free Sample
Key Developments:
Top Strategies Followed by Global Biopharmaceutical and Biomedicine Market Players
Emerging Startups - Global Biopharmaceutical and Biomedicine Market Industry Ecosystem
Biopharmaceutical and Biomedicine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 640.26 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.1% | 2032 Value Projection: | USD 1,425.17 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
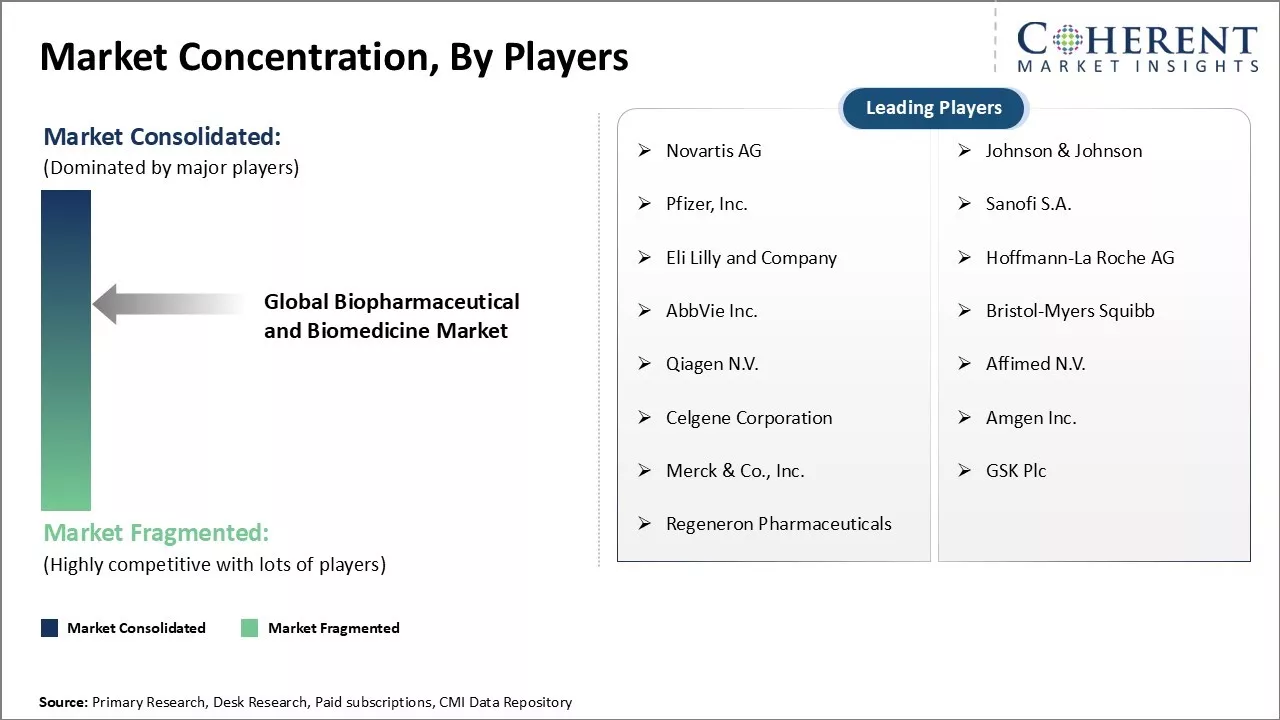
Novartis AG, Johnson & Johnson, Pfizer, Inc., Sanofi S.A., Eli Lilly and Company, Hoffmann-La Roche AG, AbbVie Inc., Bristol-Myers Squibb, Qiagen N.V., Affimed N.V., Celgene Corporation, Amgen Inc., Merck & Co., Inc., GSK Plc, and Regeneron Pharmaceuticals |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Discover market dynamics shaping the industry: Download Free Sample
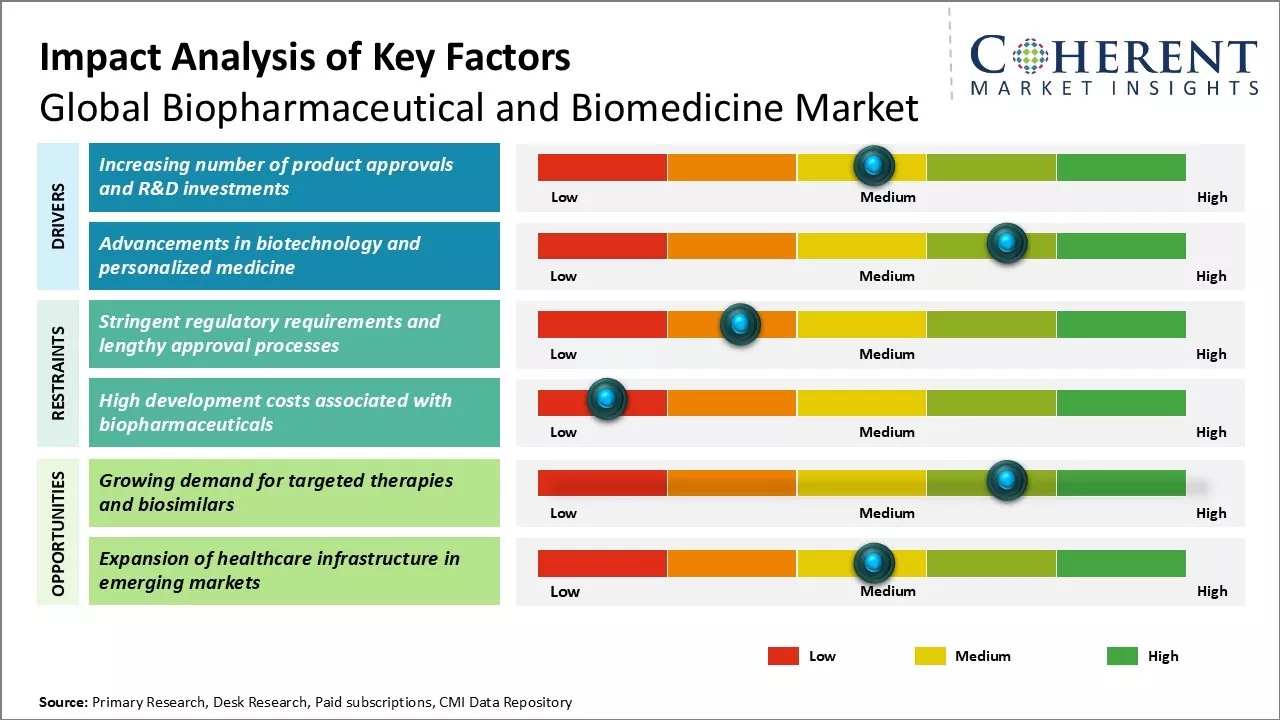
Market Driver - Increasing number of product approvals and R&D investments
The global biopharmaceutical and biomedicine market has witnessed substantial growth over the past decade owing to increasing investments in R&D by major pharmaceutical companies. There has been a consistent rise in the number of new drug approvals by regulatory authorities across the world as biopharma companies accelerate their clinical research pipelines. A key trend driving this is the large capital being pumped into the development of novel biologics and cell and gene therapies. With breakthrough discoveries in areas such as oncology, immunology, and neuroscience, many large pharmaceutical players have substantially ramped up their research budgets with the goal of bringing more innovative therapies to the market. For instance, in May 2024, Biocon Limited, a global biopharmaceutical leader, entered into an exclusive licensing and supply agreement with Handok, a specialty pharmaceutical company in South Korea, for the commercialization of Synthetic Liraglutide.
Biopharmaceutical and Biomedicine Market Challenge - Stringent regulatory requirements and lengthy approval processes
The stringent regulatory environment and lengthy approval processes for new biopharmaceutical drugs pose a major challenge for the growth of the global biopharmaceutical and biomedicine market. Most major regulatory bodies like the U.S. FDA and EMA in the EU have robust and meticulous approval systems to ensure safety and efficacy of new drugs. However, this often results in clinical trial periods lasting 7-10 years on an average before a new drug is approved. The extensive documentation and data requirements at each stage of development and regulatory review prolong the overall R&D cycle significantly. Additionally, failure in any stage leads to starting the process all over again, further draining resources and increasing costs for manufacturers. These regulatory hurdles make the drug development process highly risky, time-consuming and capital intensive, discouraging many companies especially startups. Harmonizing standards across markets and expediting approvals for breakthrough therapies can help address this challenge to some extent.
Biopharmaceutical and Biomedicine Market Opportunity - Growing demand for targeted therapies and biosimilars
The market presents robust opportunities stemming from the rising demand for biosimilars and targeted therapies. As the understanding of disease pathology improves at the molecular level, more targeted biologic drugs are being developed to precisely treat conditions. This shift from conventional drugs to precision medicines is being driven by their higher efficacy and fewer side effects. Additionally, patent expiration of major blockbuster biologics is paving the way for biosimilars which provide comparable quality at significantly lower costs. For instance, Accord BioPharma, Inc., the U.S. specialty division of Intas Pharmaceuticals, received the U.S. FDA approval for HERCESSI (trastuzumab-strf), a biosimilar to Herceptin. HERCESSI is approved for treating HER2-overexpressing breast cancer and gastric or gastroesophageal junction adenocarcinoma, marking Accord BioPharma, Inc’s first USFDA-approved biosimilar.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients