Global Abemaciclib Market Size and Forecast – 2025 to 2032
The Global Abemaciclib Market is estimated to be valued at USD 1.94 Bn in 2025 and is expected to reach USD 4.48 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.7% from 2025 to 2032. This robust growth reflects increasing adoption of Abemaciclib for targeted cancer therapies, driven by rising incidences of breast cancer and expanding approvals for its use across various oncology indications globally.
Key Takeaways of the Global Abemaciclib Market
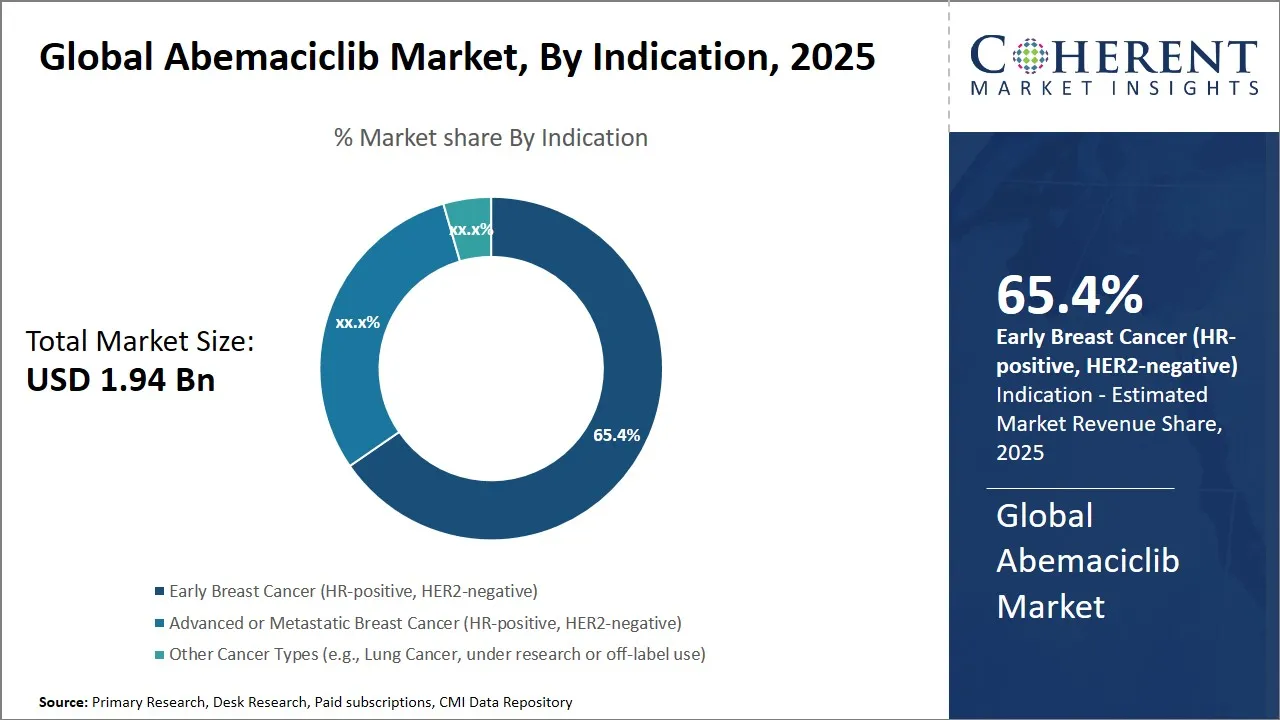
- In 2025, the early breast cancer (HR-positive, HER2-negative) segment is expected to dominate the global Abemaciclib market, accounting for 65.4% of the market share.
- The 50 mg dosage strength segment is projected to lead with a 30.3% share in 2025.
- Among the therapy type segment, monotherapy (Abemaciclib alone) is projected to hold the largest market share, contributing 79.8% in 2025.
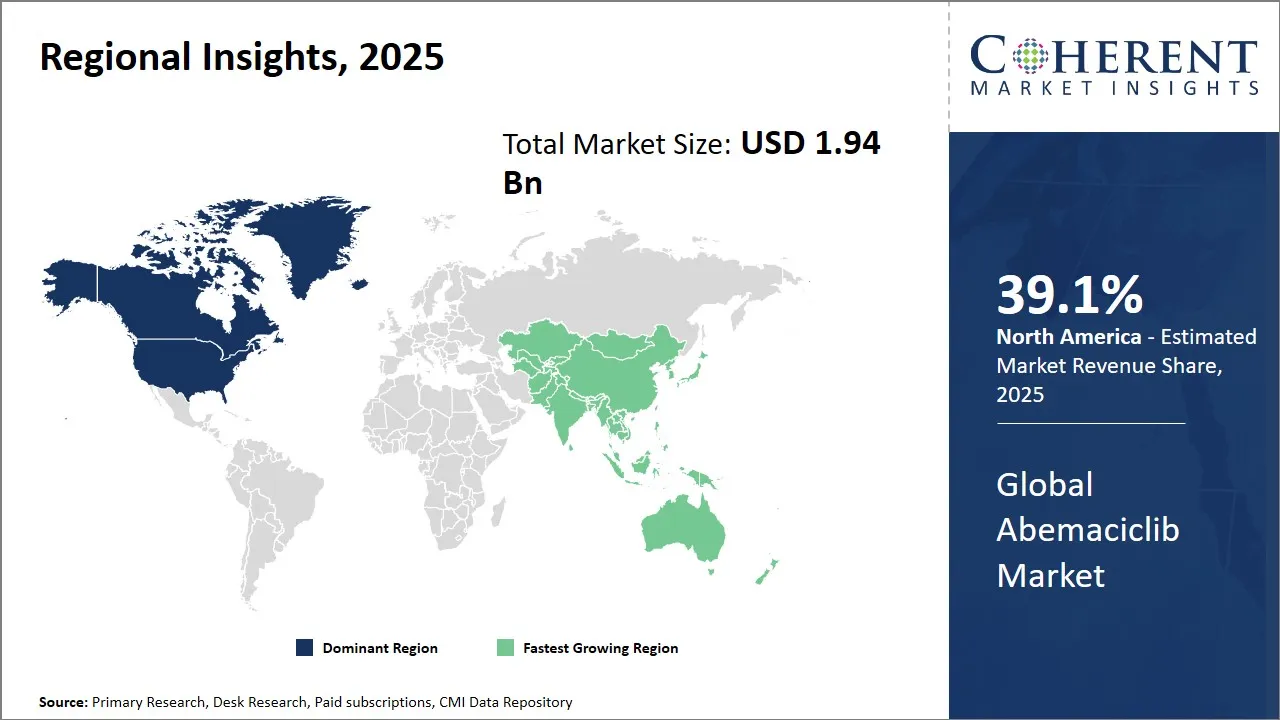
- North America is expected to lead the market, holding a share of 39.1% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with a market share of 23.5% in 2025.
Market Overview
Market trends indicate a significant shift toward personalized medicine and combination therapies involving Abemaciclib, enhancing treatment efficacy and patient outcomes. Additionally, ongoing clinical trials and advancements in biomarker research are fueling the development of next-generation CDK4/6 inhibitors, which complement Abemaciclib’s market presence. The emphasis on improved safety profiles and reimbursement policies is further boosting market penetration, especially in developed regions with advanced healthcare infrastructure.
Current Events and Its Impact
|
Current Events |
Description and its Impact |
|
Regulatory Approval in New Regions |
|
|
Expansion of Biosimilar Trastuzumab in Emerging Markets |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Abemaciclib Market Insights, By Indication - Early Breast Cancer (HR-positive, HER2-negative) is dominant due to increasing adoption of adjuvant therapies for high-risk patients and strong clinical data supporting disease-free survival benefits.
In terms of indication, Early Breast Cancer (HR-positive, HER2-negative) is going to be the leading indication in the global Abemaciclib market with an estimated 65.4% of the market share in 2025. The use of Abemaciclib has been greatly encouraged by the increasing emphasis on early intervention and adjuvant therapies, making it the main treatment in this sector.
The CDK4/6 Inhibitor, Abemaciclib that has been approved for use in patients with tumors containing HR-positive, HER2-negative breast cancer, has given sixty-six percentage of the patients improved invasive disease-free survival when taken side by side with endocrine therapy. Its mechanism of action targeting cell cycle pathways involved in tumor growth makes it a very effective treatment for minimizing relapse and improving long-term outcomes.
Abemaciclib Market Insights, By Dosage Strength - Dominance of 50 mg Dosage Strength is Driven by Safety and Personalized Therapy Needs
The 50 mg dosage strength segment of the global Abemaciclib market is predicted to hold the highest share with 30.3% in 2025, which shows that it is a central part of the process of drug therapy optimization. This dose is especially liked for starting treatment and taking care of patients whose doses are being carefully adjusted.
The dosing flexibility of Abemaciclib is such that it allows doctors to personalize the treatment according to the individual patient’s tolerability and response, with 50 mg being the starting dose for the balancing act between efficacy and adverse event management.
Abemaciclib Market Insights, By Therapy Type - Preference for Monotherapy Reflects Demand for Targeted, Simplified Cancer Treatment Regimens
Within the therapy type segmentation of the Abemaciclib market, monotherapy (Abemaciclib alone) is expected to capture the highest market share with 79.8% in 2025, signaling a clear clinical preference for targeted single-agent treatment in certain patient populations.
This trend is mainly linked to the necessity for effective yet simple treatment regimens that not only lessen drug-drug interactions but also minimize cumulative toxicities that are often found in combination therapies.
Reimbursement and Pricing Analysis of Abemaciclib
- The pricing of Abemaciclib (Verzenio) is subject to significant variability depending on the patient's insurance coverage. The list price for a 28-day supply is USD 16,330.08; however, out-of-pocket costs can range from USD 0 to USD 1,784 per month for those with employer-based insurance. Medicare Part D patients often face costs between USD 0 and USD 14 per month, with some qualifying for financial assistance programs like Extra Help, reducing their costs further. Medicaid recipients typically pay between USD 0 and USD 3 per month, depending on state regulations.
- For individuals without insurance or who have plans that do not cover Verzenio, the price is close to the list price, potentially with additional pharmacy charges. Financial assistance options, including Eli Lilly and Company’s support programs and the Lilly Cares Foundation, are available to help eligible patients access the drug at reduced or no cost. These mechanisms aim to reduce the financial burden, making Abemaciclib more accessible to patients regardless of insurance status.
Regional Insights
To learn more about this report, Download Free Sample
North America Abemaciclib Market Analysis and Trends
North America is the primary market for Abemaciclib and is expected to hold 39.1% share of the market in 2025 mainly because of the well-established pharmaceutical ecosystem and the advanced healthcare infrastructure. The presence of leading biopharmaceutical companies like Eli Lilly and Company, which was responsible for the development of Abemaciclib, is very important for the region to continue being the first in the world.
The good government policies that are in place to support oncology research, the quick drug approvals that are done by the U.S. FDA and the vast number of clinical trial activities are all factors that help to make the market very favorable for the continued growth and adoption of Abemaciclib in North America.
For instance, in June 2022, the Biden Administration, through the U.S. Department of Health and Human Services (HHS) and the Centers for Medicare & Medicaid Services (CMS), introduced the Enhancing Oncology Model (EOM) to improve cancer care for Medicare patients.
Asia Pacific Abemaciclib Market Analysis and Trends
Asia Pacific is the region showing the quickest growth in the Abemaciclib market with an estimated share of 23.5% in 2025, because of rising cancer awareness, increasing healthcare facilities, and growing investments in biopharmaceutical research in developing countries like China, India, and Japan.
Government programs to provide better healthcare access and regulatory changes to speed up drug approvals have made the market expansion easier. Also, the increasing number of breast cancer cases, the rising affordability of advanced therapies, and the increased demand for targeted cancer treatments all contribute to the quick uptake of Abemaciclib.
Abemaciclib Market Outlook for Key Countries
U.S. Abemaciclib Market Trends
The U.S. Abemaciclib market is characterized by early drug approvals, heavy R&D investments, and high-quality oncology care with Eli Lilly being the main player in the development and supply of Abemaciclib. The good reimbursement situation and the presence of clinical trials are the two factors that have given support to the adoption and growth of Abemaciclib in the U.S. oncology market.
In March 2024, Eli Lilly and Company announced it will present preclinical data on new cancer treatments at the AACR Annual Meeting in April 2024. This includes a monoclonal antibody for Nectin-4, a KRAS G12D inhibitor, and a BRM (SMARCA2) inhibitor for BRG1 mutated cancers.
Germany Abemaciclib Market Trends
Germany remains close to the top with its good healthcare system and support from the government for cancer research leading the way. Germany has established a methodical approach towards cancer treatment through the application of the evidence-based treatment protocols and health technology assessments, which will guarantee that only the patients with the certain criteria will receive Abemaciclib.
In February 2023, the German Federal Ministry of Education and Research (BMBF) expanded the National Center for Tumor Diseases (NCT) with four new sites, increasing the total to six. This expansion aims to accelerate cancer research and clinical trials, improve patient outcomes, and promote personalized oncology. The BMBF is investing USD 115.7 million annually to support this initiative, enhancing access to new treatments like Abemaciclib (Verzenio) for cancer patients across Germany.
Japan Abemaciclib Market Trends
Japan, an ongoing pioneer in the field of cancer treatment, benefits greatly from its highly developed medical and health system, and the swift incorporation of new oncological drugs. The pharmaceutical sector, backed by strong government incentives and funding for cancer research, is actively promoting the accessibility of Abemaciclib. Not only do reimbursement policies favor the innovative treatments, but they also make the drug available to the wider patient population.
In July 2025, the EU Cancer Mission and Japan’s National Cancer Centre held a joint event at the Osaka Expo focused on improving the quality of life for adolescents and young adults (AYAs) with cancer. Survivors from Europe and Japan shared stories emphasizing individualized care, less toxic treatments, and patient involvement in trials.
India Abemaciclib Market Trends
The market for abemaciclib in India is changing along with the awareness about cancer treatment and better health care systems. The government’s cancer care programs and the involvement of the private sector have made it easier for patients to get the new treatments, including Abemaciclib. Reimbursement issues are still there, but the setting up of more oncology centers and the running of clinical trials are both contributing to the market’s expansion.
In September 2025, the Ministry of Ayush inaugurated India’s first Integrative Oncology Research and Care Centre (IORCC) at the All India Institute of Ayurveda (AIIA) in Goa. The center integrates Ayurveda, Yoga, Panchakarma, and modern oncology to provide holistic, patient-centric cancer rehabilitation.
Pipeline & Strategic Collaborations Overview
|
Partner |
Nature of Collaboration |
Indication/Key Trial(s) |
Status/Strategic Notes |
|
Merck & Co., Inc. |
Immuno-oncology combination: Abemaciclib + Pembrolizumab (Keytruda) |
Multi-tumour Phase I; breast & NSCLC. |
Early stage (Phase I) in 2015. Long lead time; potential for broader tumour types. |
|
AstraZeneca plc |
Immuno-oncology/combination research: Abemaciclib with SERD (Faslodex) and other agents |
Solid tumours broad; trial design unspecified. |
Strategic alliance; non-exclusive; indicates Lilly is exploring various combinations beyond breast cancer. |
|
Veru Inc. |
Clinical trial collaboration & supply: Enobosarm + Abemaciclib in AR+ ER+ HER2- metastatic breast cancer |
Phase III ENABLAR-2 planned Q1 2022. |
Focused niche (AR+ subset) – differentiates abemaciclib’s role in resistant/targeted settings. |
|
Incyclix Bio, LLC |
Collaboration & supply agreement: INX-315 (CDK2 inhibitor) + Abemaciclib + Fulvestrant in HR+ HER2- breast cancer |
Phase 1/2 study initiation Q4 2024. |
Strategy to combine abemaciclib with next-gen cell cycle inhibitors (CDK2) to address resistance. |
|
Sermonix Pharmaceuticals, Inc. |
Combination study: Lasofoxifene + Abemaciclib in ESR1-mutant advanced breast cancer |
Phase II ELAINE-2 trial began Q3 2020. |
Focus on endocrine-resistant population; shows extension into precision medicine subsets. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- In August 2025, Eli Lilly and Company announced that its drug Verzenio (abemaciclib), when combined with endocrine therapy, significantly improved overall survival in patients with HR+, HER2, node-positive, high-risk early breast cancer, according to seven-year data from the monarchE trial. The study showed sustained benefits in invasive disease-free and distant relapse-free survival, confirming Verzenio’s two-year regimen as the standard of care.
- In March 2023, Eli Lilly and Company announced that the U.S. FDA expanded Verzenio’s (abemaciclib) use for HR+, HER2-, high-risk early breast cancer, removing the Ki-67 score requirement. Backed by the monarchE trial, Verzenio with endocrine therapy cut recurrence risk by 35% and showed over 85% four-year survival, marking a key step in oncology care and advancing safer drug desensitization practices.
- In October 2021, Quest Diagnostics, a leading U.S. provider of diagnostic information services, announced plans to launch the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) test nationwide as the first companion diagnostic for Eli Lilly and Company’s Abemaciclib (Verzenio), a CDK4/6 inhibitor approved for HR+, HER2-, high-risk early breast cancer. Following U.S. FDA approval, Quest became the first lab to validate the test with Agilent Technologies, supporting precision oncology by helping identify patients most likely to benefit from Abemaciclib-based therapy.
Market Report Scope
Abemaciclib Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.94 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.7% | 2032 Value Projection: | USD 4.48 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Eli Lilly and Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Abemaciclib Market Dynamics
To learn more about this report, Download Free Sample
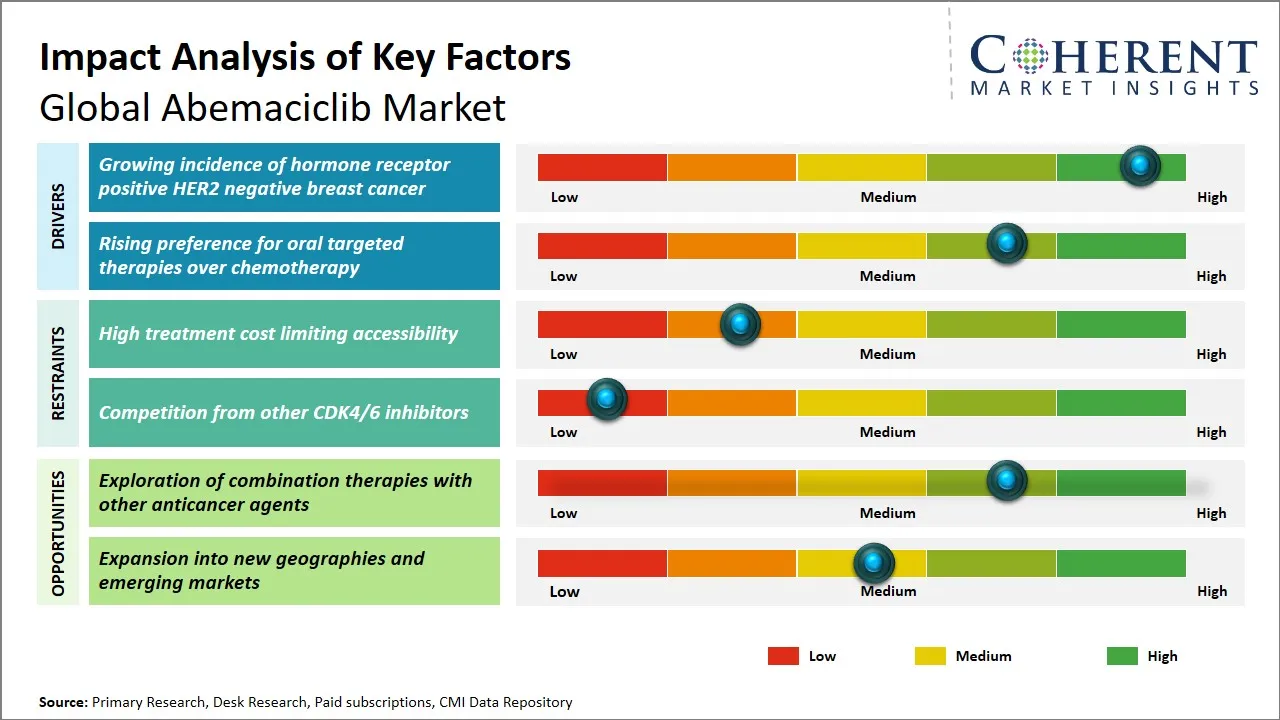
Abemaciclib Market Driver - Growing Incidence of Hormone Receptor Positive HER2 Negative Breast Cancer
Cancer that is hormone-receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) forms the main segment that is supporting the global abemaciclib market. This category is predominant among breast cancer cases and is defined by the presence of certain receptors on the cell surface, which, in turn, regulate tumor growth. The increase in awareness, detection techniques, and diagnostics has led to the misdiagnosis of HR+/HER2-patients being more accurately diagnosed, resulting in the targeted therapy, including abemaciclib, becoming more available to them.
For instance, in August 2025, according to WHO, breast cancer caused 670,000 deaths globally in 2022, with 2.3 million new cases. It affects women of all ages, with higher rates in older women. Early diagnosis and treatment are key, but significant global disparities exist in survival rates, with higher mortality in lower HDI countries.
Abemaciclib Market Opportunity - Exploration of Combination Therapies with Other Anticancer Agents
The global Abemaciclib market opens a vast area of opportunity allowing the fusion and the production of the proper combination therapies containing abemaciclib and other anticancer agents. Abemaciclib, as a selective CDK4/6 inhibitor, has been proven to work successfully in the case of hormone receptor-positive, HER2-negative breast cancer. However, at the same time, people engaged in research and clinical trials are putting their efforts into the synergistic effects of combining abemaciclib with diverse targeted therapies, immunotherapies, and chemotherapy agents so as to notch up therapeutic outcomes and fight resistance mechanisms.
In March 2025, according to the National Library of Medicine in march 2025, a Phase 1b study of abemaciclib (Eli Lilly) in combination with various therapies for metastatic breast cancer showed promising safety and efficacy. The study reported response rates of 10% to 66.7%, with the highest success seen when abemaciclib was combined with fulvestrant and LY3023414. Common side effects included diarrhea, fatigue, and neutropenia.
Analyst Opinion (Expert Opinion)
- The HR+/HER2- breast cancer is becoming more common and regulatory approvals to use Abemaciclib in both late and early-stage patients are the biggest driving forces behind the drug market. Besides, for combination therapies, Abemaciclib is becoming an essential medicine with longer progression-free periods and wider patient base being created. The oral administration of the drug and its real-world effectiveness are also factors that support its use. On the other hand, issues that will have to be dealt with are the price cuts, the management of the side effects in the new and broader patient populations and the resistance that is likely to develop as the use of the drug is extended.
- The American Society of Clinical Oncology (ASCO) 2023, along with the San Antonio Breast Cancer Symposium 2024, has highlighted the importance of knowledge-sharing. As a result, Abemaciclib was widely discussed as the treatment of choice in both early and metastatic stages. Among one of the real-world actions that have taken place, there are the studies conducted in Denmark demonstrating that patients who received Abemaciclib first-line have better outcomes and the regulatory approvals for early breast cancer treatment given to several countries.
Market Segmentation
- Indication Insights (Revenue, USD Bn, 2020 - 2032)
- Early Breast Cancer (HR-positive, HER2-negative)
- Advanced or Metastatic Breast Cancer (HR-positive, HER2-negative)
- Other Cancer Types (e.g., Lung Cancer, under research or off-label use)
- Dosage Strength Insights (Revenue, USD Bn, 2020 - 2032)
- 50 mg
- 100 mg
- 150 mg
- 200 mg
- Therapy Type Insights (Revenue, USD Bn, 2020 - 2032)
- Monotherapy (Abemaciclib alone)
- Combination Therapy (with endocrine therapy, other cancer drugs)
- Line of Therapy Insights (Revenue, USD Bn, 2020 - 2032)
- First-line endocrine-based treatment
- Second-line after progression on prior endocrine therapy
- Third-line and later after chemotherapy
- Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Academic oncology centers
- Comprehensive cancer hospitals
- Private oncology clinics
- Government/public hospitals
- Others (home care)
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Eli Lilly and Company
Sources
Primary Research Interviews
- Industry Stakeholders
- Oncology drug‑development executives at major CDK4/6 inhibitor firms
- Regulatory affairs lead in oncology pharmaceuticals
- End‑users
- Oncologists specializing in HR+/HER2‑ breast cancer treatment
- Hospital pharmacy directors in major cancer centers
- Health‑economics / outcomes‑research professionals analyzing CDK4/6 usage
- Patient advocacy group representatives for breast‑cancer therapies
Government & International Databases
- U.S. Food and Drug Administration (FDA) Drug Approvals & Databases
- National Cancer Institute (NCI) “Drugs Approved for Different Types of Cancer” database
- Central Drugs Standard Control Organisation (India) List of Approved New Drugs
- ClinicalTrials.gov (U.S.) database of clinical studies and results
- OECD/WHO pharmaceutical statistics (generic)
- EU‑EMA (European Medicines Agency)‑public approval and pharmacovigilance register
Trade Publications
- Pharmaceutical trade magazine reports on oncology drug launches
- Specialty oncology market newsletters covering CDK4/6 inhibitors
- Industry journal “PharmaVOICE” or equivalent covering drug‑launch trends
- Quarterly oncology drug pricing/reimbursement summaries in trade press
- Specialty newsletters: oncology formulary access, biosimilar competition
- Biopharma regulatory affairs trade publications
Academic Journals
- Journal of Managed Care & Specialty Pharmacy
- Tandfonline - Clinical studies on Abemaciclib for treating HR+/HER2‑
- National Institutes of Health (NIH) clinical research articles
- Journal of Clinical Oncology (JCO) updates on Abemaciclib in adjuvant therapy
- MDPI journals – Real-world efficacy of Abemaciclib in clinical settings
- Journal of Cancer Research and Clinical Oncology (JCRCO)
Reputable Newspapers
- Financial Times
- The Wall Street Journal
- The New York Times
- The Guardian
- Reuters Health
- Bloomberg Healthcare
Industry Associations
- American Society of Clinical Oncology (ASCO)
- British Pharmacological Society (BPS)
- International Society of Oncology Pharmacy Practitioners (ISOPP)
- International Society for Pharmacoeconomics and Outcomes Research (ISPOR)
- Association for Human Pharmacology in the Pharmaceutical Industry (AHPPI)
- Cancer Drug Development Forum (CDDF)
Public Domain Resources
- WHO Global Health Observatory (GHO) statistics on cancer incidence
- World Bank health‑expenditure data
- NIH/NCI cancer‑treatment summaries and fact sheets
- Open‑access datasets for oncology drug approvals & timelines
- Government health‑department open data portals (e.g., U.S., EU, India)
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients