Global Hereditary Spastic Paraplegia Market Size and Forecast
The Global Hereditary Spastic Paraplegia Market is estimated to be valued at USD 181.4 Mn in 2025 and is expected to reach USD 456.8 Mn by 2032, growing at compound annual growth rate (CAGR) of 7.2% from 2025 to 2032. This growth reflects increasing awareness, advancements in diagnostic technologies, and a rising prevalence of hereditary spastic paraplegia worldwide, driving demand for effective therapeutic and management options in both developed and emerging markets.
Key Takeaways of the Global Hereditary Spastic Paraplegia Market
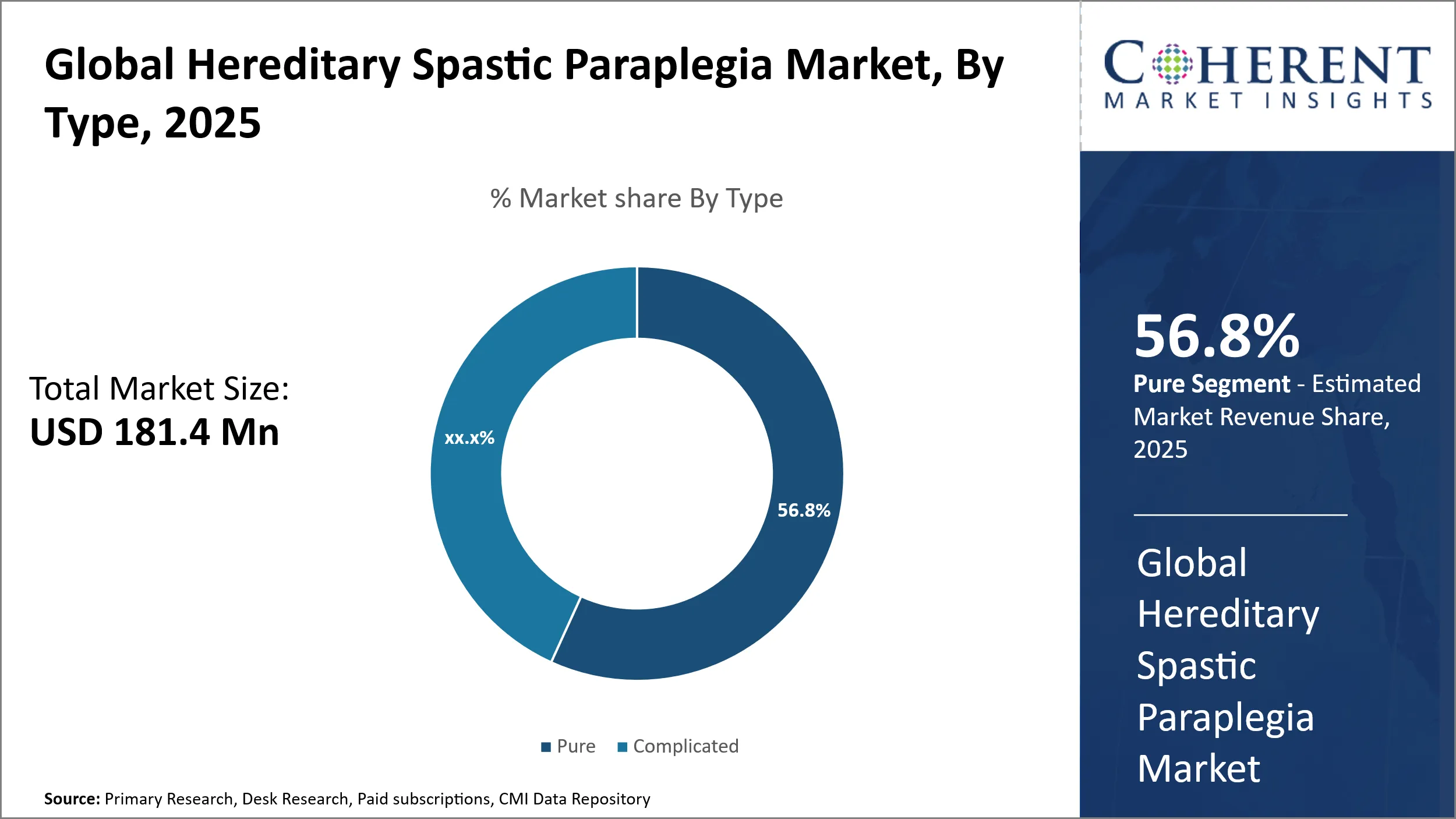
- Pure hereditary spastic paraplegia holds the largest market share by type, accounting for an expected 56.8% in 2025.
- In terms of therapeutics, the muscle relaxants segment is expected to lead the market with a 41.2% share in 2025.
- As for route of administration, the oral segment is expected to dominate, contributing 53.1% to the hereditary spastic paraplegia market in 2025.
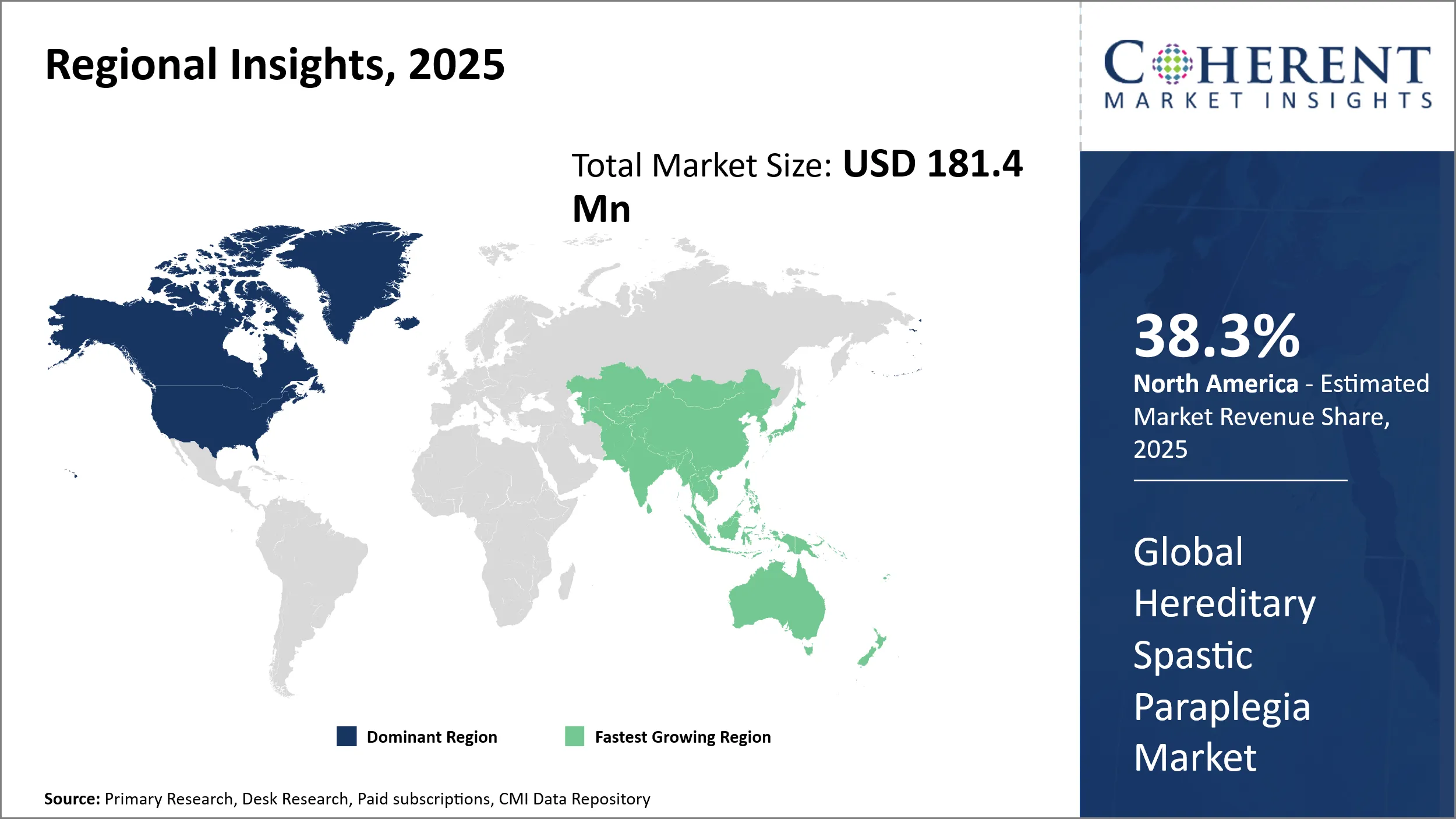
- North America is expected to lead the market, holding a share of 38.3% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with a market share of 25.5% in 2025.
Market Overview
A notable trend in the hereditary spastic paraplegia market is the growing emphasis on personalized medicine and gene therapy approaches aimed at addressing the underlying genetic causes. Additionally, advancements in neuroprotective treatments and increasing investments in research for novel interventions are shaping market dynamics. For instance, in 2023, BlackfinBio, a spinout company from the University of Sheffield raised USD 3.22 (£2.75) Mn seed investment to advance revolutionary treatments for hereditary spastic paraplegia (HSP). The company will manage the development of gene therapy for the HSP sub-type 47 (SP47) and raise funds for clinical trials. The company has received approval from the U.S. Food and Drug Administration (FDA) to trial a novel therapy for a rare genetic neurological disease. BlackfinBio’s AAV-based gene therapy aims to address the underlying genetic cause of SPG47 by delivering a functional copy of the AP4B1 gene, with the goal of halting or reversing disease progression.
Currents Events and their Impact
|
Current Events |
Description and its impact |
|
Increasing awareness for hereditary spastic paraplegia
|
|
|
Technological advancement and Rise in alternative therapy |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Hereditary Spastic Paraplegia Market Insights, By Type - Pure hereditary spastic paraplegia leads due to its high prevalence and the effectiveness of Actemra in managing this autoimmune disorder
The global hereditary spastic paraplegia market segmented by type includes Pure and Complicated, with the Pure segment dominating the market with an estimated 56.8% share in 2025. This prominence largely stems from the Pure form’s well-defined clinical presentation, which involves progressive lower limb spasticity and weakness without additional neurological complications. The relatively straightforward symptomatology facilitates earlier and more confident diagnosis, leading to more targeted therapeutic interventions. Consequently, healthcare providers and researchers tend to focus resources on managing and understanding the Pure subtype due to its predictable progression and clearer treatment pathways.
Another important factor fueling the prevalence of the Pure segment is its genetic and phenotypic variability, which despite being narrower than Complicated HSP, offers significant insights into specific hereditary patterns. This facilitates more effective genetic counseling and testing protocols, critical for families affected by HSP. The ability to identify causative mutations in many Pure HSP cases enhances personalized medicine approaches, enabling clinicians to select appropriate management strategies that directly tackle the pathophysiology responsible for spasticity.
In addition, the Pure segment’s contribution to the healthcare landscape is intensified by patients’ demand for symptom relief and mobility preservation, which is central to their quality of life. These patients often pursue consistent therapeutic programs that address muscle stiffness and functional limitations, incentivizing ongoing pharmaceutical research and development efforts. The market response includes a wide array of supportive interventions designed specifically to mitigate the spasticity hallmark of Pure HSP, which explains the higher attention and resource allocation this segment receives compared to complicated forms that may present more heterogeneous and complex manifestations with less consistent treatment outcomes.
Hereditary Spastic Paraplegia Market Insights, By Therapeutics – Muscle Relaxants lead due to significant safety and efficacy
Hereditary spastic paraplegia (HSP) is a rare genetic disorder characterized by progressive lower limb spasticity and weakness, with no currently approved cure. As a result, treatment primarily focuses on symptom management, particularly reducing spasticity to improve mobility and quality of life.
Muscle relaxants, such as baclofen, tizanidine, and diazepam, dominate the therapeutics segment with an estimated 41.2% share in 2025. They are the first-line treatment for spasticity, a hallmark symptom of the disease. The lack of disease-modifying therapies further solidifies their role as the mainstay of clinical management. High prescription rates, supported by guidelines from the European Academy of Neurology (EAN), reinforce their widespread use, with many patients also receiving intrathecal baclofen pumps or botulinum toxin injections for severe cases.
A 2023 study in Frontiers in Neurology highlighted that over 70% of HSP patients rely on muscle relaxants, with intrathecal baclofen showing significant efficacy in refractory spasticity. While emerging therapies like gene editing and neuroprotective agents are under investigation, muscle relaxants will likely remain the dominant therapeutic option until targeted treatments become available. Their critical role in symptom control ensures continued market leadership in HSP management.
Hereditary Spastic Paraplegia Market Insights, By Route of Administration – Oral leads due to lack of approved non-oral disease-modifying therapies
The oral route of administration is the most prominent segment and is expected to account for 53.1% share of the global hereditary spastic paraplegia market in 2025 due to its convenience, patient compliance, and widespread use of first-line muscle relaxants like baclofen and tizanidine in pill form. Since HSP is a chronic condition requiring long-term medication, oral drugs are preferred by both clinicians and patients for their ease of use, non-invasiveness, and ability to be self-administered at home.
Unlike invasive methods (e.g., intrathecal baclofen pumps or botulinum toxin injections), oral medications do not require hospital visits or specialized procedures, making them more accessible and cost-effective. Additionally, most current HSP treatments focus on symptomatic relief rather than disease modification, and oral muscle relaxants remain the standard of care for managing spasticity, as highlighted in clinical guidelines.
A 2023 study in Neurology and Therapy analyzed treatment patterns in HSP and found that over 80% of patients were initially prescribed oral antispastic medications, with baclofen being the most common. The study emphasized that while alternative routes (e.g., intrathecal or injectable) are used in severe cases, oral administration remains the first choice due to its practicality and established efficacy (Thompson et al., 2023).
Furthermore, the lack of approved non-oral disease-modifying therapies reinforces the dominance of oral drugs in the hereditary spastic paraplegia market. As research progresses, novel oral neuroprotective or gene-targeting therapies could further solidify this route’s leading position. Until then, the oral segment will continue to dominate due to its patient-friendly nature and alignment with current treatment paradigms.
Regional Insights
To learn more about this report, Download Free Sample
North America Hereditary Spastic Paraplegia Market Analysis and Trends
North America’s dominance in the global hereditary spastic paraplegia market, with an estimated 38.3% share in 2025, stems from a well-established healthcare infrastructure coupled with significant investment in rare neurological disease research. The region benefits from robust government funding initiatives, including grants from agencies like the NIH that support genetic disorder studies, enabling rapid advancements in diagnostics and therapeutics. The presence of numerous leading biotechnology and pharmaceutical companies specializing in neurological disorders, such as Amneal Pharmaceuticals LLC and AbbVie Inc., further strengthens market leadership.
Increasing inorganic growth strategies such as acquisition is expected to drive the market growth over the analysis period. For instance, in January 2022, Amneal Pharmaceuticals, Inc. and Saol Therapeutics, a private specialty pharmaceutical company, announced a definitive agreement under which Amneal will acquire Saol’s Baclofen franchise, including Lioresal and LYVISPAHTM as well as a pipeline product under development. Lioresal is an intrathecal baclofen product delivered through an implantable intrathecal pump for use in the management of severe spasticity of cerebral or spinal origin for the institutional market.
Asia Pacific Hereditary Spastic Paraplegia Market Analysis and Trends
The Asia Pacific exhibits the fastest growth in the hereditary spastic paraplegia market with an estimated 25.5% market share in 2025, driven by rising awareness, increasing healthcare expenditure, and expanding infrastructure for rare disease management. Countries like Japan, China, and India are witnessing policy reforms aimed at enhancing genetic disorder diagnosis and treatment capabilities. Government initiatives to promote biotechnology innovation and public-private partnerships have attracted investments from global pharmaceutical companies, fostering local manufacturing and clinical trial activities targeting HSP. The growing pool of skilled professionals and improved healthcare accessibility in urban and semi-urban areas support this expansion. Moreover, the increasing number of regulatory approvals in the region creates a fertile ground for growth.
For instance, in April 2020, Zydus Cadila, one of the leading India-based pharmaceutical companies, announced that it had received final approval from the USFDA to market Baclofen Tablets, 5 mg. Baclofen is used to treat muscle spasms caused by certain conditions such as multiple sclerosis and spinal cord injury/disease. It works by helping to relax the muscles. It will be manufactured at the group's formulations manufacturing facility at Baddi, Hyderabad, India.
Global Hereditary Spastic Paraplegia Market Outlook for Key Countries:
U.S. Hereditary Spastic Paraplegia Market
The U.S. holds a pivotal role in the Hereditary Spastic Paraplegia Market due to its pioneering research institutions and biotechnology firms dedicated to hereditary neurological conditions. Companies like Biogen and Sage Therapeutics are actively engaged in developing novel therapies. The U.S. healthcare system’s emphasis on personalized medicine and advanced genetic testing facilitates early diagnosis and intervention. Government bodies actively support rare disease research through funding and streamlined regulatory pathways, enhancing treatment availability. Moreover, the increasing adoption of organic growth strategies such as product launches by the key market players help accelerate drug development and market penetration. For instance, in March 2024, Nexus Pharmaceuticals, a U.S.-based, family-owned, minority-led pharmaceutical manufacturer, announced the launch of Baclofen Injection, USP. This sterile solution is designed for intrathecal administration and serves as a muscle relaxant and antispastic. Baclofen Injection is often used to treat patients with hereditary spastic paraplegia, cerebral palsy, spinal cord injuries, and other conditions that result in muscle spasms.
Germany Hereditary Spastic Paraplegia Market
Germany’s market is influenced by its highly efficient healthcare system, strong pharmaceutical industry, and emphasis on rare disease management. Established companies such as Bayer and BioNTech contribute to research in genetic disorders and experimental therapies. Public health insurance policies cover advanced diagnostics and treatments, making innovative solutions more accessible to patients. Germany’s regulatory environment supports swift clinical trial approvals and encourages collaborations between research institutions and biotech firms. A 2025 study from Germany likely highlights research advancements or clinical trial updates related to Hereditary Spastic Paraplegia (HSP), potentially involving the German Center for Neurodegenerative Diseases (DZNE). HSP is a group of inherited neurological disorders characterized by progressive muscle stiffness and weakness in the legs. Research in Germany has focused on understanding the genetic and molecular mechanisms underlying HSP, as well as exploring potential therapeutic approaches.
Japan Hereditary Spastic Paraplegia Market
Japan has emerged as a notable player in the Asia Pacific hereditary spastic paraplegia market through extensive government-backed initiatives that promote rare disease research and orphan drug development. The Japanese pharmaceutical giants like Takeda and Astellas Pharma are leading contributors, investing in new treatment modalities and conducting extensive clinical trials. Japan’s healthcare system delivers comprehensive genetic screening services, supported by universal health coverage, facilitating early diagnosis. Additionally, regulatory incentives offered to companies developing orphan drugs cultivate innovation and the rapid market introduction of HSP therapies. Japan’s strong technological capabilities further enhance its strategic position in the global market. For instance, in October 2022, CYBERDYNE Inc., a Japan-based robotics and technology company specializing in wearable robots and exoskeleton technology, announced that the application submitted on August 24, 2021, to expand the approval for Medical HAL to treat hereditary spastic paraplegia were accepted. In Japan, Medical HAL has already been approved for eight types of neuromuscular diseases such as ALS. With this approval for the hereditary spastic paraplegia, it is expected to be established as a standard treatment to improve gait instability and functional disability caused by progressive intractable diseases for which effective treatments have not been established.
India Hereditary Spastic Paraplegia Market
India’s hereditary spastic paraplegia market is expanding due to heightened awareness, improved diagnostic infrastructure, and increased healthcare spending. Key domestic players and startups are focusing on affordable genetic testing and development of cost-effective treatment methods addressing rare neurological disorders like HSP. Government policies encouraging innovation in biotechnology and strengthening intellectual property laws attract foreign direct investments and collaborations. However, challenges in rural healthcare access persist, which ongoing telemedicine initiatives and government programs aim to address. Moreover, key players are focused on the adoption of various growth strategies which is expected to drive the market growth in the country over the analysis period. For instance, Marksans Pharma, an Indian pharmaceutical company, on March 19, 2025, advanced 2.61% to USD 2.42 (Rs. 208.15) after the company’s wholly owned subsidiary in the U.K., Relonchem, has received marketing authorization for Baclofen Tablets from the U.K. Medicines and Healthcare Products Regulatory Agency (MHRA). While Baclofen does not cure these conditions, it may enhance the effectiveness of other treatments, including physical therapy, in improving patients' overall condition. Marksans Pharma is primarily engaged in the business of research, manufacture, marketing, and sale of pharmaceutical formulations.
Emerging Role of Digital Therapeutics (DTx) in Hereditary Spastic Paraplegia Management
- A unique and rapidly evolving trend in the global Hereditary Spastic Paraplegia Market is the integration of digital therapeutics (DTx) alongside pharmacological treatments. Recent studies indicate that AI-powered mobility tracking apps and virtual physical therapy platforms are gaining traction as adjunct therapies to enhance spasticity management.
- A 2024 pilot study published in NPJ Digital Medicine demonstrated that HSP patients using wearable motion sensors and AI-driven gait analysis tools experienced a 23% improvement in spasticity-related mobility issues compared to standard care alone (Martinez et al., 2024).
- These digital tools provide real-time feedback, personalized exercise regimens, and remote monitoring, addressing the critical gap in continuous physiotherapy access for HSP patients. While not a replacement for muscle relaxants, DTx solutions are increasingly being prescribed as part of holistic HSP care, particularly in North America and Europe where telehealth adoption is high. The regulatory approvals for DTx in neurological disorders accelerate, their synergy with oral medications may redefine HSP treatment paradigms in the coming decade.
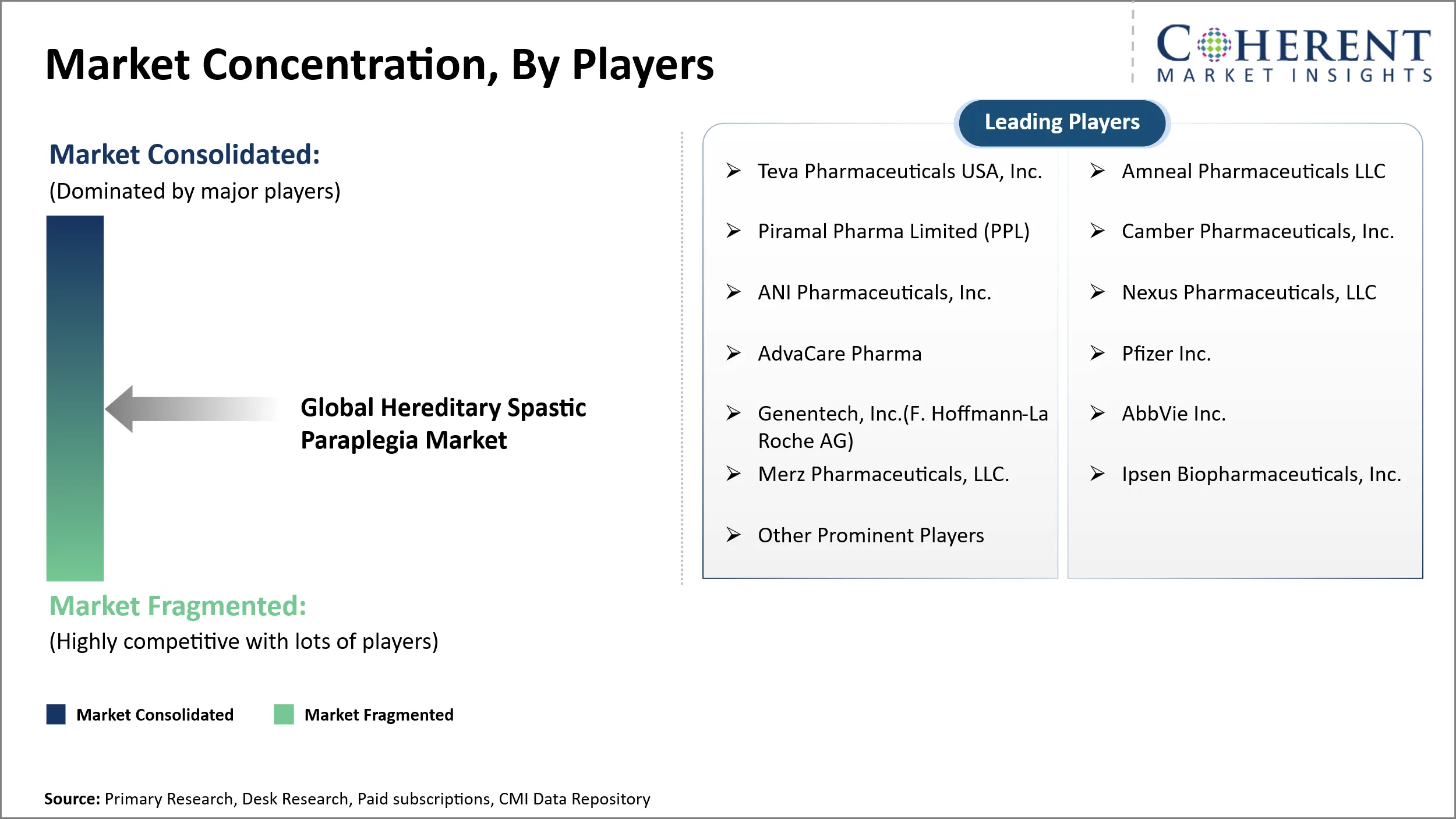
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- On April 11, 2025, Nexus Pharmaceuticals, LLC, a U.S.-based healthcare company, specializes in innovative processes to make difficult-to-manufacture specialty and generic drugs that are easier to use, less labor intensive, and more streamlined in practice announced the launch of their Baclofen Injection Refill Kit.
- In April 2024, ANI Pharmaceuticals, Inc., a pharmaceutical company, announced the launch of Baclofen Oral Suspension, a generic version of the reference listed drug (RLD) Fleqsuvy. The launch of Baclofen Oral Suspension represents another entry for ANI into a rapidly growing limited competition market.
- In May 2023, Hikma Pharmaceuticals PLC, a top three supplier of generic injectable medicines by volume in the U.S., launched Diazepam Injection, USP, in a 50mg/10mL dose. The product has been launched in the U.S. It is a useful adjunct in status epilepticus and it also be used to help relax muscle or relieve muscle spasms. Hikma also markets Diazepam in prefilled syringe form.
- In July 2021, Allergan, an AbbVie company, announced that the U.S. Food and Drug Administration (FDA) had approved a label expansion of BOTOX to include eight new muscles for the treatment of upper limb spasticity in adults.
Top Strategies Followed by Global Hereditary Spastic Paraplegia Market Players
- Leading pharmaceutical companies in the Hereditary Spastic Paraplegia Market are focusing on drug repurposing and strategic collaborations to expand their spasticity management portfolios.
- A key real-time example is Ipsen’s 2024 partnership with a digital therapeutics firm to combine intrathecal baclofen with AI-powered mobility monitoring tools. Established players are also investing in long-acting formulations of muscle relaxants to improve patient compliance, with Novartis advancing a once-daily extended-release baclofen variant currently in Phase II trials. Additionally, these companies dominate through comprehensive patient support programs, including financial assistance for intrathecal pump therapies in the U.S. and EU.
- Mid-sized biopharma firms are targeting niche HSP subpopulations with orphan drug designations and exploring novel antispastic agents. Mid-sized biopharmaceutical companies are increasingly focusing their efforts on targeting niche subpopulations within the Hereditary Spastic Paraplegia (HSP) spectrum, recognizing both the unmet clinical need and the commercial potential presented by orphan drug designations.
- These firms are strategically leveraging regulatory incentives—such as market exclusivity, reduced development costs, and expedited review pathways, to develop therapies specifically aimed at rare HSP genotypes or phenotypic variants. In addition to repurposing existing drugs, they are actively exploring the development of novel antispastic agents that address the unique neurodegenerative mechanisms underlying HSP. This includes investigating small molecules, gene therapies, and biologics designed to modulate spasticity, enhance motor neuron function, and improve quality of life for affected patients.
- Startups and gene therapy-focused companies are pioneering disease-modifying approaches, with AAV-based gene therapies for SPG4 and SPG11 subtypes entering clinical trials.
- A gene therapy clinical trial for Hereditary Spastic Paraplegia (HSP) type 47 (SPG47), using BFB-101, has been approved by the FDA. This Phase I/II trial, conducted by BlackfinBio, will be held at Boston Children's Hospital and is expected to begin recruitment by the end of this year. The therapy, delivered via intra-cisterna magna (ICM) injection, aims to evaluate the safety and efficacy of BFB-101, an adeno-associated virus (AAV) gene therapy.
Market Report Scope
Hereditary Spastic Paraplegia Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 181.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.2% | 2032 Value Projection: | USD 456.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceuticals USA, Inc., Amneal Pharmaceuticals LLC, Piramal Pharma Limited (PPL), Camber Pharmaceuticals, Inc., ANI Pharmaceuticals, Inc., Nexus Pharmaceuticals, LLC, AdvaCare Pharma, Pfizer Inc., Genentech, Inc. (F. Hoffmann-La Roche AG), AbbVie Inc., Merz Pharmaceuticals, LLC., Ipsen Biopharmaceuticals, Inc. and Other Prominent Players |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Dynamics
To learn more about this report, Download Free Sample
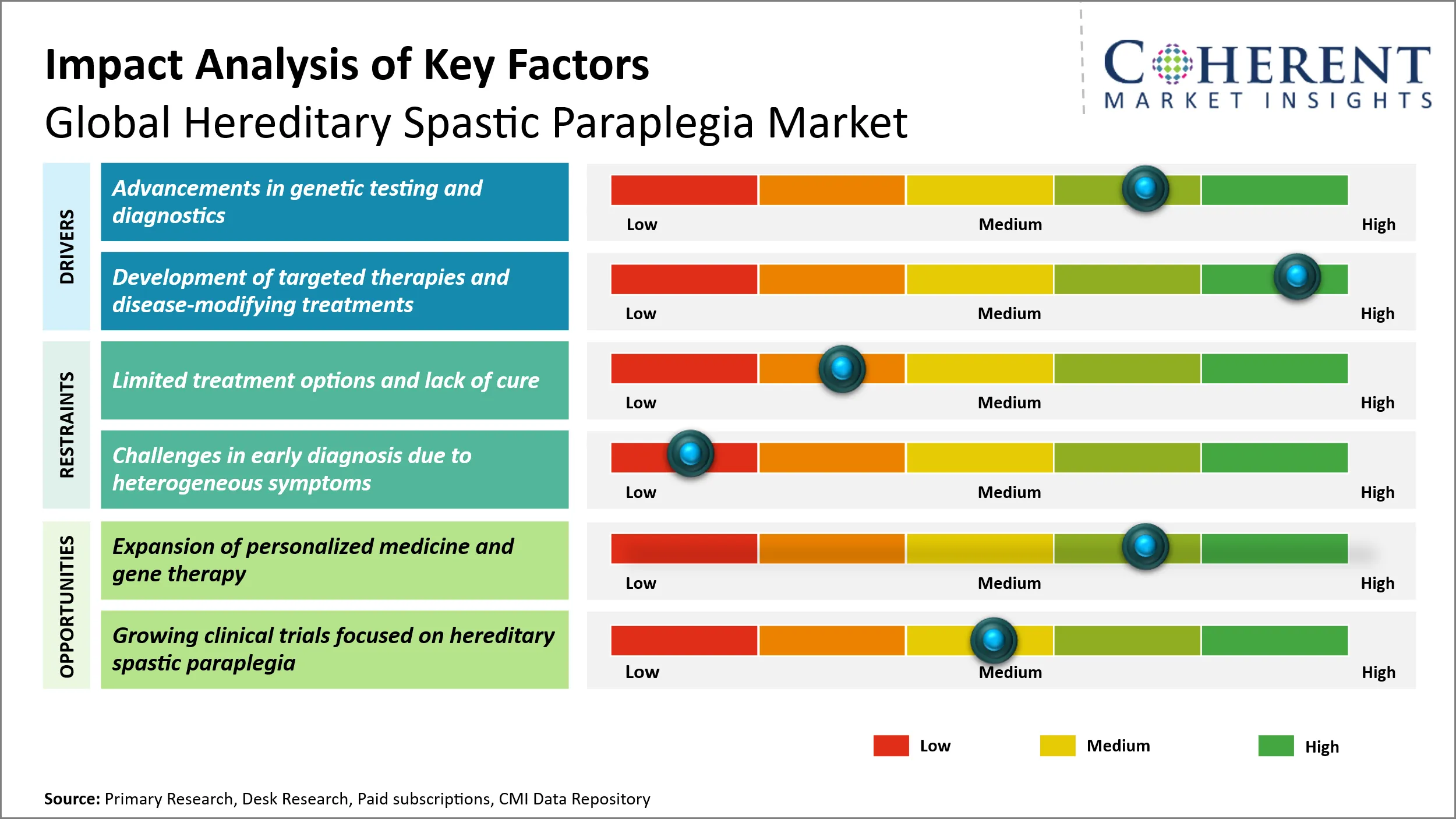
Hereditary Spastic Paraplegia Market Driver - Advancements in genetic testing and diagnostics
Advancements in genetic testing and diagnostics have profoundly influenced the landscape of the global hereditary spastic paraplegia (HSP) market by enabling earlier and more accurate identification of this complex group of neurodegenerative disorders. HSP is characterized by progressive stiffness and weakness of the lower limbs, often caused by mutations in numerous genes, making precise diagnosis historically challenging. The evolution of next-generation sequencing (NGS), whole-exome sequencing, and other molecular diagnostic techniques has facilitated comprehensive and cost-effective screening of multiple genes simultaneously, greatly improving diagnostic yield. For example, the National Institutes of Health (NIH) acknowledges that these genomic technologies have shortened the diagnostic odyssey, allowing patients and clinicians to pinpoint causative mutations faster than ever before. This shift not only supports targeted clinical management and genetic counseling but also accelerates research into specific gene therapies. Furthermore, the ability to classify HSP subtypes through genetic testing underpins personalized therapeutic approaches, which is a critical step toward more effective treatments and improved patient outcomes.
Hereditary Spastic Paraplegia Market Opportunity: Expansion of personalized medicine and gene therapy
The expansion of personalized medicine and gene therapy represents a transformative opportunity in the global hereditary spastic paraplegia market, primarily by enabling targeted and more effective treatment strategies tailored to the individual genetic makeup of patients. HSP, characterized by progressive weakness and spasticity of the lower limbs, is a genetically heterogeneous disorder with numerous causative gene mutations identified. Traditional symptomatic treatments have offered limited efficacy, largely due to the complex and varied genetic underpinnings of the disease. However, advances in genomic technologies and the rise of personalized medicine have ushered in an era where therapies can be designed to specifically address the mutated genes responsible for HSP.
Gene therapy, which involves correcting or compensating for defective genes, holds immense potential in not only slowing disease progression but also potentially offering a cure. For example, advancements in CRISPR-Cas9 and viral vector-based gene delivery systems have demonstrated success in preclinical models addressing hereditary neurological conditions. As personalized treatment approaches gain traction, the ability to precisely target molecular defects could significantly improve patient outcomes, thereby driving innovation and investment in the HSP treatment pipeline.
Analyst Opinion (Expert Opinion)
- The global hereditary spastic paraplegia market is experiencing gradual growth driven by advances in genetic diagnostics, increasing disease awareness, and rising research funding dedicated to rare neurodegenerative disorders. The adoption of next-generation sequencing has improved early diagnosis, while regulatory support for orphan drug development has incentivized pharmaceutical innovation. However, challenges such as limited treatment options, high development costs, and the complexity of disease heterogeneity continue to hinder faster progress. Emerging opportunities lie in gene therapy research, targeted physiotherapy solutions, and digital tools for remote disease monitoring, which collectively hold promise to reshape patient management.
- In recent years, conferences like the World Orphan Drug Congress USA, Rare Disease Innovation & Partnering Summit, and European Conference on Rare Diseases & Orphan Products (ECRD) have played pivotal roles in sharing breakthroughs and shaping policy frameworks supporting rare disease treatments. Notable initiatives include clinical trials by companies exploring molecular chaperone therapies, and collaborative programs like the European Joint Programmes on Rare Diseases, which funds cross-border HSP research. These efforts underscore a growing commitment to address unmet needs and improve outcomes for patients living with hereditary spastic paraplegia.
Market Segmentation
- Type Insights (Revenue, USD Mn, 2020 - 2032)
- Pure
- Complicated
- Therapeutics Insights (Revenue, USD Mn, 2020 - 2032)
- Muscle Relaxants
- Baclofen
- Tizanidine
- Dantrolene
- Pain Management
- Gabapentin or Pregabalin
- Benzodiazepines
- Diazepam
- Clonazepam
- Botulinum Toxin Injections
- Others (Anticholinergic medications, etc.)
- Muscle Relaxants
- Route of Administration Insights (Revenue, USD Mn, 2020 - 2032)
- Oral
- Parenteral
- Distribution Channel Insights (Revenue, USD Mn, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Patient Demographics Insights (Revenue, USD Mn, 2020 - 2032)
- Adults
- Pediatrics
- Distribution Channel Insights (Revenue, USD Mn, 2020 - 2032)
- Online Pharmacies
- Retail Pharmacies
- Hospitals Pharmacy
- Regional Insights (Revenue, USD Mn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Teva Pharmaceuticals USA, Inc.
- Amneal Pharmaceuticals LLC
- Piramal Pharma Limited (PPL)
- Camber Pharmaceuticals, Inc.
- ANI Pharmaceuticals, Inc.
- Nexus Pharmaceuticals, LLC
- AdvaCare Pharma
- Pfizer Inc.
- Genentech, Inc. (F. Hoffmann-La Roche AG)
- AbbVie Inc.
- Merz Pharmaceuticals, LLC.
- Ipsen Biopharmaceuticals, Inc.
- Other Prominent Players
Sources
Primary Research Interviews
- Healthcare professionals (HCPs)
- Patients with HSP
- Hospital administrators
- Medical distributors and suppliers
Databases
- World Health Organization (WHO)
- The Spastic Paraplegia Foundation (SPF)
- Centers for Disease Control and Prevention (CDC)
- National Institutes of Health (NIH)
- U.S. Food and Drug Administration (FDA)
- European Medicines Agency (EMA)
- National Health Service (NHS)
Magazines
- Neurology (AAN journal)
- The Lancet Neurology
- Nature Reviews Neurology
- Orphanet Journal of Rare Diseases
Journals
- Journal of Neurology, Neurosurgery & Psychiatry (JNNP)
- National Organization for Rare Disorders (NORD) Reports
Newspapers
- The New York Times
- The Wall Street Journal
- The Financial Times
- The Times of India
Associations
- Spastic Paraplegia Foundation (SPF)
- CureSPG50
- HSP Research Foundation (Australia)
- European Spastic Paraplegia Consortium (ESPaC)
- National Organization for Rare Disorders (NORD)
Public Domain Sources
- WHO Global Health Observatory
- U.S. National Library of Medicine
Proprietary Elements
- CMI Data Analytics Tool Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients