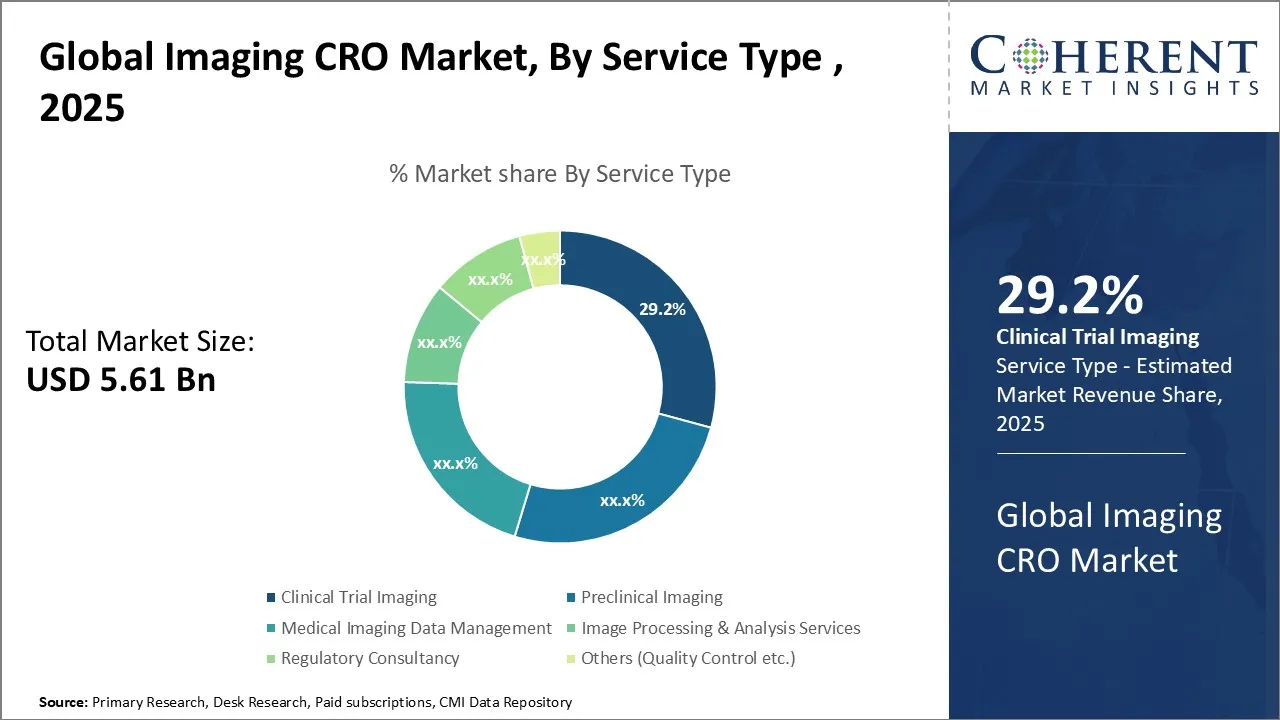
Global Imaging CRO Market Size and Forecast – 2025-2032
The Global Imaging CRO Market is estimated to be valued at USD 5.61 Bn in 2025 and is expected to reach USD 9.68 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.1% from 2025 to 2032. This significant growth can be attributed to the increasing demand for imaging services in clinical trials, rising prevalence of chronic diseases, and growing adoption of advanced imaging technologies in the healthcare industry.
Key Takeaways of the Global Imaging CRO Market
- By service type, clinical trial imaging is expected to account for the largest market share, representing 29.2% in 2025.
- Magnetic Resonance Imaging (MRI) is projected to lead the market with a 32.2% share in 2025.
- In terms of therapeutic area, oncology is expected to dominate the global imaging CRO market, capturing a 33.4% share in 2025.
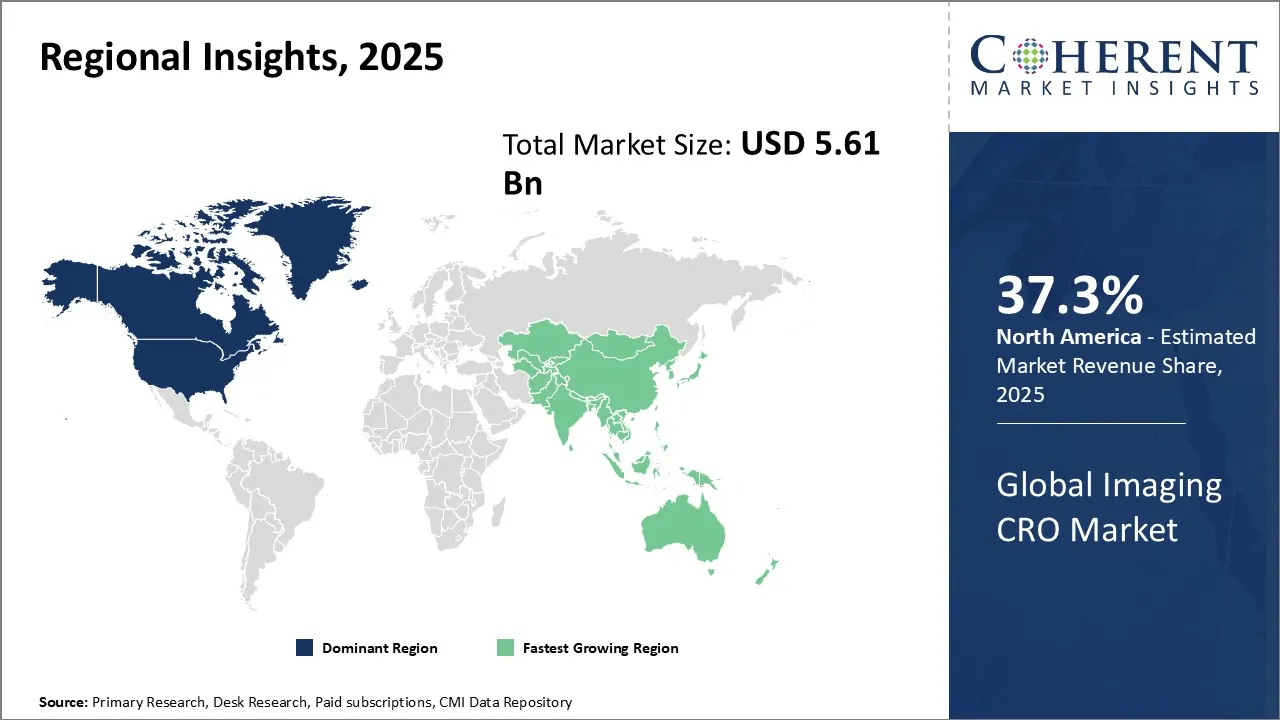
- North America is expected to lead the market, holding a share of 37.3% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with a market share of 34.2% in 2025.
Market Overview
The imaging CRO market trend is characterized by the increasing outsourcing of imaging services by pharmaceutical and biotechnology companies to specialized imaging contract research organizations. This trend is driven by the need for cost-effective and efficient imaging solutions, as well as the growing complexity of clinical trials involving imaging endpoints. Additionally, the market is witnessing a shift towards the use of advanced imaging modalities, such as positron emission tomography (PET) and magnetic resonance imaging (MRI), which provide higher resolution and more accurate data compared to traditional imaging techniques.
Current Events and Its Impact
|
Current Events |
Description and its impact |
|
Emergence of Advanced Imaging Modalities |
|
|
Growth in Oncology and Neurology Clinical Trials |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Imaging CRO Market Insights, By Service Type - Advancements in Clinical Trial Imaging Propel the Segment Growth
In terms of service type, clinical trial imaging is expected to contribute the highest share of the market with a share of 29.2% in 2025 owing to the increasing demand for advanced imaging technologies in clinical trials. The growing complexity of clinical trials and the need for precise and reliable imaging data have driven the adoption of innovative imaging solutions in the pharmaceutical and biotechnology industries.
Clinical trial imaging plays a crucial role in the drug development process, enabling researchers to visualize and assess the efficacy and safety of novel therapeutics. The use of advanced imaging modalities, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), allows for non-invasive evaluation of drug distribution, metabolism, and pharmacodynamic effects in real-time.
Imaging CRO Market Insights, By Imaging Modality - Magnetic Resonance Imaging (MRI) Dominates as it Emerges as the Imaging Modality of Choice
In terms of imaging modality, Magnetic Resonance Imaging (MRI) is expected to contribute the highest share of the market with a share of 32.2% in 2025. MRI has emerged as a preferred imaging modality in various therapeutic areas due to its superior soft-tissue contrast, non-invasive nature, and absence of ionizing radiation.
The versatility of MRI in providing high-resolution anatomical images, as well as functional and physiological information, has made it an indispensable tool in clinical trials. MRI enables the visualization of structural abnormalities, tumor characterization, and assessment of treatment response in oncology trials. In neurological studies, MRI allows for the evaluation of brain structure, function, and connectivity, facilitating the understanding of neurological disorders and the development of targeted therapies.
Imaging CRO Market Insights, By Therapeutic Area
-Oncology Leads the Way in Therapeutic Area Dominance
Oncology dominates the global imaging CRO market due to the increasing prevalence of cancer and advancements in diagnostic technologies with an estimated share of 33.4% in 2025. Cancer remains one of the leading causes of death worldwide, prompting pharmaceutical and biotech companies to prioritize oncology clinical trials. Imaging plays a critical role throughout the cancer patient journey—beginning with initial diagnosis and staging, where modalities like PET-CT, MRI, and CT provide detailed insights into tumor size, location, and spread. Imaging is also central to treatment planning, helping clinicians select appropriate therapies and monitor their effectiveness.
In oncology trials, imaging biomarkers are essential for assessing tumor response to treatments, such as changes in size or metabolic activity, which are crucial endpoints for regulatory approval and trial success. Imaging also enables longitudinal monitoring to detect disease progression, recurrence, or metastases, and evaluate therapy-related toxicities. Furthermore, innovations like AI-driven imaging analysis, radiomics, and advanced molecular tracers enhance the precision and reproducibility of imaging data, supporting personalized medicine and targeted therapies.
Role of Artificial Intelligence (AI) in the Global Imaging CRO Market
- Artificial Intelligence (AI) is fundamentally reshaping the global Imaging CRO market, unlocking significant value through enhanced efficiency, cost optimization, and accelerated drug development timelines. AI-driven technologies such as machine learning algorithms, deep learning models, and computer vision systems are transforming imaging data analysis by enabling automated interpretation, anomaly detection, and predictive analytics. These capabilities not only reduce human error but also speed up the clinical trial process, thereby lowering costs and improving accuracy in image-based endpoints. AI’s role extends to process automation—optimizing image processing pipelines, reducing manual workloads, and enabling real-time data insights that support faster decision-making.
- For instance, Median Technologies, a France-based Imaging CRO, has successfully integrated AI into its iBiopsy platform, which develops non-invasive imaging biomarkers and predictive algorithms to support oncology trials. This approach helps in earlier and more accurate detection of therapeutic responses, offering a competitive edge in precision medicine. However, companies must balance the benefits of AI with challenges like data quality issues, regulatory hurdles, and the need for skilled talent to ensure AI outputs are reliable and clinically actionable.
Regional Insights
To learn more about this report, Download Free Sample
North America Imaging CRO Market Analysis and Trends
North America’s dominance in the global imaging CRO market with an estimated share of 37.3% in 2025 can be attributed to several factors. The region boasts a robust healthcare infrastructure, advanced technology adoption, and a strong presence of major pharmaceutical and biotechnology companies. The United States, in particular, has been at the forefront of medical imaging research and development, with a supportive regulatory environment and substantial investments in clinical trials. North America's market ecosystem fosters collaboration between imaging CROs, sponsors, and healthcare providers, enabling efficient and innovative imaging solutions.
Government policies and initiatives, such as the National Institutes of Health (NIH) funding for medical research, have further propelled the growth of the imaging CRO sector in the region. For Instance, on May 1, 2025, Alimentiv, a global GI-focused CRO offering clinical trials, imaging, and precision medicine services, partnered with Dova Health Intelligence (formerly Satisfai Health), a U.S.-based AI healthcare software company, to launch DovaVision at DDW 2025 in California, U.S. DovaVision is an AI-powered endoscopy analysis tool designed for ulcerative colitis (UC) clinical trials.
Asia Pacific Imaging CRO Market Analysis and Trends
The Asia Pacific region is expected to exhibit the fastest growth in the global imaging CRO market with a share of 34.2% in 2025. This growth can be ascribed to the region's rapidly expanding healthcare sector, increasing clinical trial activities, and growing demand for advanced medical imaging services. Countries like China, India, and Japan have made significant strides in developing their healthcare infrastructure and attracting global pharmaceutical companies to conduct clinical trials in the region. The Asia Pacific's large patient population, coupled with cost-effective clinical trial operations, has made it an attractive destination for imaging CROs.
Government initiatives to promote healthcare innovation, such as China's "Healthy China 2030" plan and Japan's "Asia Health and Wellbeing Initiative," have further fueled the market's growth. Additionally, the presence of skilled medical professionals and the adoption of advanced imaging technologies have contributed to the region's rapid expansion. Key players, such as WuXi AppTec and Pharmaron, have emerged as significant contributors to the Asia Pacific imaging CRO landscape.
Global Imaging CRO Market Outlook for Key Countries
U.S. Imaging CRO Market Trends
The U.S. market holds a dominant position in the global imaging CRO market. The country's advanced healthcare system, robust research and development infrastructure, and significant investments in medical imaging technologies have been key drivers of growth. The presence of leading pharmaceutical and biotechnology companies, along with renowned academic and research institutions, has fostered a strong market ecosystem.
Government support, through funding agencies like the National Institutes of Health (NIH), has further propelled the adoption of imaging CROs in clinical trials. For instance, in April 2023, Mednet, a healthcare technology firm, launched a new Imaging module within its iMednet eClinical platform, enabling seamless DICOM image upload, storage, and review for clinical trials.
China Imaging CRO Market Trends
China's imaging CRO market has witnessed remarkable growth in recent years, driven by the country's expanding healthcare sector and increasing focus on medical research and development. The Chinese government's initiatives, such as the "Made in China 2025" plan, have prioritized the development of advanced medical technologies, including medical imaging. The country's large patient population and growing clinical trial activities have attracted global imaging CROs to establish their presence in China.
For instance, in January 2025, XingImaging, a leader in advanced research imaging and radiopharmaceutical services, opened a cutting-edge research facility in U.S. The new site features cGMP radiochemistry labs, a dedicated clinical research clinic, PET-CT and SPECT-CT scanners, and advanced image analysis capabilities. With a focus on precision-driven scientific services and technologies like the NeuroExplorer PET scanner, XingImaging aims to support the research community in developing life-saving treatments.
Japan Imaging CRO Market Trends
Japan continues to be a prominent player in the global imaging CRO market, renowned for its technological advancements and high-quality healthcare services. The country's aging population and increasing prevalence of chronic diseases have driven the demand for advanced medical imaging solutions. Japan's regulatory environment, which emphasizes safety and efficacy, has fostered a robust market for imaging CROs. The country's strong research and development capabilities, coupled with collaborations between academia and industry, have further propelled market growth.
For instance, in September 2022, Crown Bioscience, a global preclinical CRO, and MBL, a Japanese life sciences company, launched their joint venture—Crown Bioscience & MBL—to provide full-service drug discovery and development capabilities in Japan. This partnership offers Japanese biopharmaceutical companies’ local access to Crown’s expertise in preclinical and translational research, including organoids, high-content imaging, and an extensive PDX model collection.
Germany Imaging CRO Market Trends
Germany's imaging CRO market stands out in Europe, driven by the country's strong healthcare infrastructure, advanced medical technology, and significant investments in research and development. Germany's regulatory framework, which prioritizes patient safety and data integrity, has created a favorable environment for imaging CROs. The country's well-established pharmaceutical industry and collaborations with academic institutions have further fueled market growth.
For instance, in March 2023, Clario, a healthcare research technology company, in Germany providing endpoint solutions for clinical trials, introduced a cloud-based Image Viewer tool that simplifies and accelerates image access for sponsors and CROs. The intuitive, data privacy-compliant platform provides real-time, on-demand access to full-resolution images, eliminating delays and streamlining the imaging process for cost-effective trials across therapeutic areas.
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- On May 6, 2025, XingImaging, a MITRO company specializing in advanced imaging technologies and radiopharmaceutical services, launched the NX PET Camera, a cutting-edge PET imaging system designed to accelerate biomarker research in Parkinson’s disease. The camera offers high-resolution imaging, AI-driven data analysis, and advanced biomarker mapping to enhance the detection and tracking of neurodegenerative changes
- In March 2025, Telix Pharmaceuticals, a precision medicine company, received U.S. FDA approval for Gozellix (TLX007-CDx), a cold kit for preparing gallium-68 (68Ga) gozetotide injection for PET imaging of PSMA-positive lesions in prostate cancer patients. Gozellix offers enhanced accessibility with a longer shelf life of up to 6 hours and is intended for patients with suspected metastases or recurrence.
- In January 2025, GE HealthCare, a global leader in medical technology and digital solutions, partnered with the University of California, San Francisco (UCSF) to establish the Care Innovation Hub. The collaborative research initiative aims to advance medical imaging accessibility, non-invasive diagnosis, and precision oncology. Based at UCSF facilities in the U.S., the hub will focus on developing automated, patient-specific imaging techniques and optimizing healthcare delivery to support equitable and personalized care.
- In November 2023, MCRA, a leading independent medical device, diagnostics, and biologics CRO and advisory firm, introduced its AI & Imaging Center – the industry’s first integrated solution supporting the entire medical device product lifecycle.
Top Strategies Followed by Global Imaging CRO Market Players
- Established players in the global imaging CRO market are focusing on extensive research and development to innovate high-performance products. These companies are investing heavily in R&D to stay ahead of the competition and maintain their market dominance.
- In October 2024, Proscia, a digital pathology software company, has launched Concentriq Embeddings and the Proscia AI Toolkit to help life sciences organizations accelerate biomarker discovery, optimize clinical trials, and develop companion diagnostics. Integrated into the Concentriq platform, the tools provide AI developers and researchers access to advanced pathology models, enabling rapid in silico experiments and streamlined AI deployment in routine workflows.
- Mid-level players in the global imaging CRO market are adopting strategies focused on delivering cost-effective solutions to target price-sensitive consumers. They are striving to provide quality products at competitive prices to attract a wider customer base.
- In March 2023, Clario, a healthcare research technology company providing endpoint solutions for clinical trials, has introduced a cloud-based Image Viewer tool that simplifies and accelerates image access for sponsors and CROs.
- Small-scale players in the global imaging CRO market are targeting niche markets by offering unique features or innovative products. They are specializing in specific market segments to differentiate themselves from larger competitors. They are also forming strategic partnerships with major industry players and original equipment manufacturers (OEMs) to solidify their market presence.
- In August 2021, Fluidigm, a leading biotechnology tools provider advancing health insights, partnered with ImaBiotech, a contract research organization offering spatial multi-omics services, to expand Imaging Mass Cytometry (IMC) capabilities for drug development.
Market Report Scope
Imaging CRO Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 5.61 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.1% | 2032 Value Projection: | USD 9.68 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Siemens Healthineers, GE Healthcare, Philips Healthcare, Canon Medical Systems, Fujifilm Holdings Corporation, Agfa-Gevaert Group, Hitachi Medical Corporation, Carestream Health, Hologic, Inc., Toshiba Medical Systems, Mindray Medical International Limited, Varian Medical Systems, Esaote S.p.A., Bracco Imaging S.p.A., and Leica Biosystems |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Imaging CRO Market Dynamics
To learn more about this report, Download Free Sample
Imaging CRO Market Driver - Increasing demand for advanced imaging technologies
The global imaging CRO market is witnessing a significant driver in the form of increasing demand for advanced imaging technologies. As medical science progresses and the need for precise diagnostic tools intensifies, healthcare providers and pharmaceutical companies are actively seeking cutting-edge imaging solutions. These advanced technologies, such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT), offer detailed insights into the human body's structure and function. The adoption of these sophisticated imaging modalities enables researchers to gain a deeper understanding of disease pathology, drug efficacy, and treatment response.
Moreover, the growing prevalence of chronic diseases and the rising geriatric population further fuel the demand for advanced imaging technologies. As a result, imaging CROs are experiencing a surge in demand for their specialized services, including image acquisition, analysis, and interpretation, thereby driving the growth of the global imaging CRO market. For instance, in February 2023, GE Healthcare, a global leader in medical technology announced an agreement to acquire Caption Health, Inc., a privately held provider of artificial intelligence (AI) in healthcare solutions. Caption Health specializes in developing AI-powered clinical applications that assist in early disease detection and enhance the accuracy and efficiency of ultrasound imaging.
Imaging CRO Market Opportunity - Growth in Personalized Medicine
The increasing focus on personalized medicine presents a significant opportunity for the Global Imaging CRO Market. Personalized medicine aims to tailor medical treatments to the individual characteristics of each patient, taking into account their genetic profile, lifestyle, and environment. Imaging plays a crucial role in personalized medicine, as it enables the visualization and quantification of biological processes at the molecular and cellular levels. Imaging techniques, such as functional MRI, PET, and single-photon emission computed tomography (SPECT), can provide valuable insights into disease progression, treatment response, and drug target engagement.
The growth in personalized medicine is expected to drive the demand for advanced imaging services, as pharmaceutical and biotechnology companies seek to develop targeted therapies and companion diagnostics. Imaging CROs with expertise in specialized imaging techniques and biomarker development will be well-positioned to capitalize on this opportunity. Furthermore, the increasing collaboration between imaging CROs, pharmaceutical companies, and academic institutions in the field of personalized medicine will foster innovation and accelerate the development of personalized treatment strategies. In October 2024, Crown Bioscience, a global contract research organization (CRO) headquartered in California, U.S and part of JSR Life Sciences and JICC, has expanded its Singapore facility with cutting-edge oncology models and imaging technologies. Located in Asia’s biomedical hub, the facility now offers advanced capabilities such as MRI for orthotopic and systemic models, IVIS, MiXeno human immunity platform, and comprehensive biomarker services.
Analyst Opinion (Expert Opinion)
- The global imaging CRO market is witnessing robust growth, driven by technological advancements such as AI-powered imaging analytics, the proliferation of hybrid imaging modalities, and the increasing adoption of imaging biomarkers in clinical trials. Regulatory support from agencies like the FDA and EMA, recognizing the role of imaging endpoints in accelerating drug development, further fuels market expansion. Additionally, the rising demand for precision medicine and oncology trials, coupled with the growing pipeline of novel therapeutics requiring complex imaging protocols, creates a strong tailwind for the sector. However, the market faces challenges, including the high cost of advanced imaging technologies, regulatory complexities across regions, and the need for standardized imaging protocols globally. Emerging opportunities lie in decentralized clinical trials, AI-based image interpretation, and partnerships between CROs, pharma companies, and imaging tech providers to streamline imaging workflows.
- Key events such as the European Congress of Radiology (ECR), Radiological Society of North America (RSNA) Annual Meetings, and the World Molecular Imaging Congress have played pivotal roles in shaping the imaging CRO landscape by showcasing innovations in imaging technology, discussing policy and regulatory updates, and fostering collaborations between stakeholders. Real-world examples include partnerships like Parexel’s collaboration with Medidata for imaging data management and the establishment of global imaging networks by ICON and Bioclinica. Government initiatives like the UK’s Imaging Network for Clinical Trials and the US FDA’s guidance on imaging standards in drug development have further catalyzed the market, encouraged harmonization and advancing the role of imaging in accelerating therapeutic approvals.
Market Segmentation
- Service Type Insights (Revenue, USD Bn, 2020 - 2032)
- Clinical Trial Imaging
- Preclinical Imaging
- Medical Imaging Data Management
- Image Processing & Analysis Services
- Regulatory Consultancy
- Others (Quality Control, etc.)
- Imaging Modality Insights (Revenue, USD Bn, 2020 - 2032)
- Magnetic Resonance Imaging (MRI)
- Computed Tomography (CT)
- Positron Emission Tomography (PET)
- Ultrasound
- X-ray
- Optical Imaging
- Therapeutic Area Insights (Revenue, USD Bn, 2020 - 2032)
- Oncology
- Neurology
- Cardiology
- Musculoskeletal Disorders
- Gastroenterology
- Endocrinology
- Others
- Clinical Trial Phase Insights (Revenue, USD Bn, 2020 - 2032)
- Phase I
- Phase II
- Phase III
- Phase IV
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Device Manufacturers
- Diagnostic Centers
- Contract Research Organizations
- Academic and Research Institutes
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Siemens Healthineers
- GE Healthcare
- Philips Healthcare
- Canon Medical Systems
- Fujifilm Holdings Corporation
- Agfa-Gevaert Group
- Hitachi Medical Corporation
- Carestream Health
- Hologic, Inc.
- Toshiba Medical Systems
- Mindray Medical International Limited
- Varian Medical Systems
- Esaote S.p.A.
- Bracco Imaging S.p.A.
- Leica Biosystems
Sources
Primary Research Interviews
- Senior executives and management teams at leading imaging CROs
- Clinical trial investigators and site managers
- Regulatory affairs specialists in pharmaceutical and medical device companies
- Radiologists and imaging technologists involved in clinical trials
- Contract research organizations’ project managers and study directors
- Academic researchers and subject matter experts in medical imaging
Databases
- U.S. Food and Drug Administration (FDA)
- European Medicines Agency (EMA)
- National Institutes of Health (NIH)
- World Health Organization (WHO)
- Centers for Medicare & Medicaid Services (CMS)
- Organisation for Economic Co-operation and Development (OECD)
Magazines
- Applied Clinical Trials
- Outsourcing-Pharma.com
- Clinical Leader
- CenterWatch Weekly
- BioPharma Dive
Journals
- The Lancet Oncology
- Journal of Clinical Oncology
- Journal of Magnetic Resonance Imaging (JMRI)
- Journal of Nuclear Medicine
- Academic Radiology
- Journal of Digital Imaging
Newspapers
- The New York Times (Science/Health Section)
- The Guardian (Health Section)
- The Washington Post (Health & Science)
- The Financial Times (Pharma and Healthcare Coverage)
Associations
- Society for Clinical Research Sites (SCRS)
- Association of Clinical Research Organizations (ACRO)
- Society for Imaging Informatics in Medicine (SIIM)
- European Society of Radiology (ESR)
- Radiological Society of North America (RSNA)
Public Domain Sources
- World Bank Open Data
- UNESCO Institute for Statistics
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients