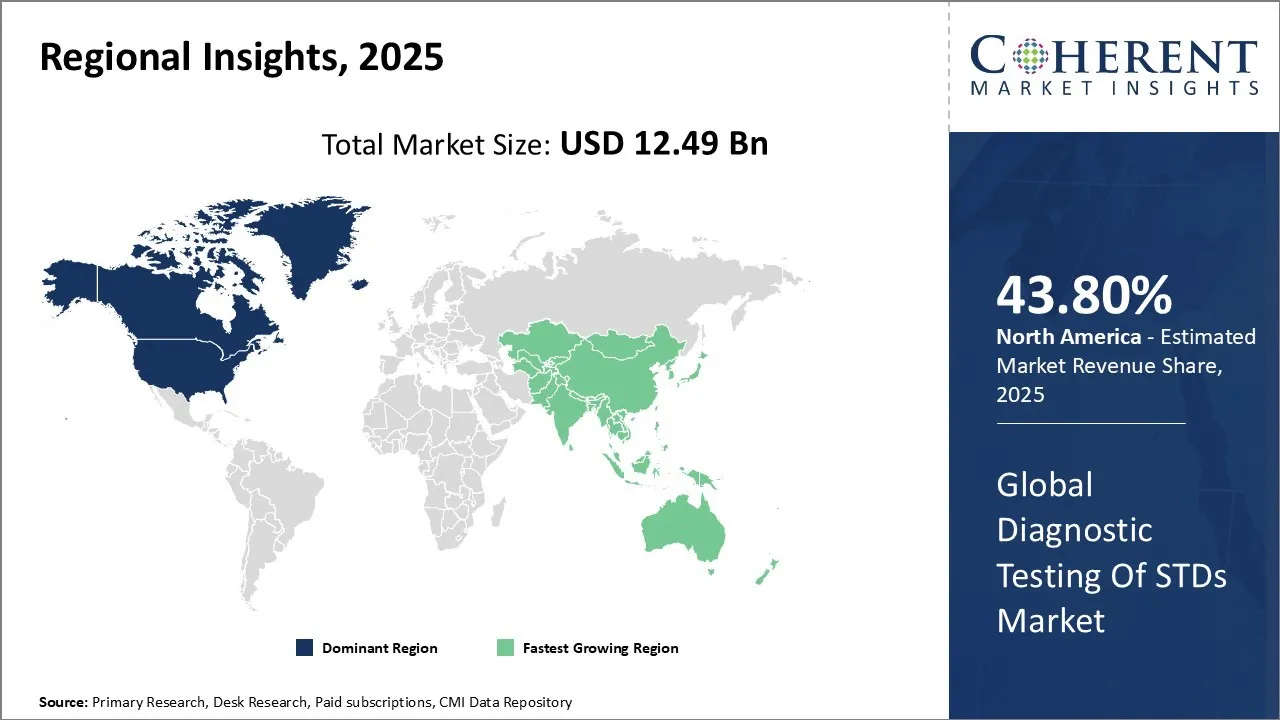
Diagnostic Testing of STDs Market size is estimated to be valued at USD 12.49 Bn in 2025 and is expected to reach USD 23.73 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of9.6% from 2025 to 2032.
The global diagnostic testing of STDs market demand is driven by the high prevalence of asymptomatic infections, the potential for serious health complications, and the risk of transmission to others. Many individuals with STIs don't experience symptoms, making regular testing essential for early detection and treatment. Diagnostic testing for STDs (sexually transmitted diseases) involves various methods, including blood tests, urine tests, and swab tests, to detect infections. The specific test used depends on the suspected infection and may involve analyzing blood, urine, or fluid samples from sores. Some STDs are diagnosed through blood tests (like HIV or syphilis), while others, like chlamydia and gonorrhea, can be detected via urine or swab tests. The STD tests are conducted are various settings including healthcare provider's offices, clinics, and even at home.
|
Current Event |
Description and its Impact |
|
Regulatory Approvals and Technological Advancements |
|
|
Antimicrobial Resistance and Diagnostic Innovation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
CMS provides reimbursement for screening and diagnostic testing of certain sexually transmitted infections (STIs) based on the recommendations of the U.S. Preventive Services Task Force (USPSTF). Key aspects of the CMS coverage policy include:
In terms of test type, the chlamydia segment is expected to contribute the 41.3% share in the market in 2025, primarily due to the suboptimal sensitivity and specificity of some diagnostic methods, the asymptomatic nature of many infections, and the need for specialized testing procedures, especially for certain populations. Furthermore, the potential for serious complications if left untreated and the associated costs of managing those complications contribute to the perceived burden of testing. Chlamydia diagnostic testing involves analyzing samples (usually urine or swabs from the genital area, throat, or rectum) to detect the presence of the bacteria Chlamydia trachomatis.
In March 2025, the U.S. FDA authorized the Visby Medical Women’s Sexual Health Test, the first over-the-counter, prescription-free, fully at-home diagnostic for chlamydia, gonorrhea, and trichomoniasis. It is designed for users with or without symptoms, the single-use test delivers results in approximately 30 minutes using a self-collected vaginal swab and a portable device linked to the Visby app.
To learn more about this report, Download Free Sample
North America is estimated to hold a dominant position in the global diagnostic testing of STDs market over the forecast period owing to increasing product launches. For instance, Bio-Rad Laboratories, Inc., a U.S.-based manufacturer of life science technology, entered into a partnership with Seegene Inc., a South Korea-based diagnostic products manufacturer for the development and commercialization of infectious disease diagnostic products. Under the terms of the agreement, Seegene Inc. will provide diagnostic tests for use on Bio-Rad’s CFX96 Dx Real-Time PCR System for the U.S. market. Additionally, North America has government initiatives promoting early detection and treatment. For instance, the U.S. Centers for Disease Control and Prevention (CDC) has unveiled its STD PCHD (Prevention and Control for Health Departments) program, allocating more than USD 7 million to fortify STD infrastructure nationwide. This initiative supports STI monitoring, testing, treatment, data-driven public health action, and targeted interventions in high-burden populations across states and large metropolitan areas. It complements EHE (Ending the HIV Epidemic) funding totaling $593 million—to expand PrEP access and incorporate doxycycline PEP strategies.
Asia Pacific is considered to be the fastest growing region during the forecast period. The region’s growth is driven by rising infection rates, a large and growing population, increasing awareness, and advancements in testing technologies. Countries like China and India have massive populations, contributing significantly to the overall number of STD cases in the region. There were 6.7 million people living with HIV in 2023 in Asia region.
In May 2025, Elyon Family Clinic & Surgery expanded its diagnostic services by introducing two new sexually transmitted disease (STD) testing packages in Singapore, targeting enhanced screening and early detection efforts. The first package includes rapid testing for common infections such as chlamydia, gonorrhea, and HIV, while the second adds advanced diagnostics like syphilis RPR, hepatitis B and C, and HPV screening. Both packages are designed for privacy, convenience, and same-day results. In August 2024, Andhra Pradesh State Aids Control Society (APSACS) launched an intensified IEC (Information, Education and Communication) campaign to raise awareness among the public on HIV/AIDS and other sexually transmitted diseases (STDs).
The U.S. diagnostic testing of STDs market is characterized by the asymptomatic nature of many STIs, and the potential for serious health complications if left untreated. Additionally, advancements in testing technologies, like rapid point-of-care tests, have increased accessibility and convenience, further driving demand. According to the CDC’s 2024 surveillance data, the U.S. recorded over 2.5 million cases of chlamydia, gonorrhea, and syphilis combined the highest in decades. Syphilis alone rose by over 17% in 2024, with congenital syphilis increasing by 30%, prompting nationwide alerts. This alarming trend has made diagnostic testing for STDs a public health priority, further contributing to the diagnostic testing of STDs market demand.
The diagnostics testing of STDs market demand in Japan due to the 2024 policy expansion mandating broader syphilis testing. The policy now requires routine treponemal testing, including TPPA (Treponema pallidum particle agglutination) assays, across a wider population, including pregnant women, high-risk groups, and routine health checkups in metropolitan areas such as Tokyo and Osaka. This shift responds to the alarming surge in syphilis cases 2023 saw over 12,000 cases nationally, the highest since post-war records began. The expanded testing framework is forecasted to create a USD 120 million incremental market, with demand growing at over 20% annually through 2026. Diagnostic companies are scaling up production and distribution of rapid and lab-based TPPA kits to meet this need. Hospitals and clinics are integrating automated syphilis panels into their diagnostic workflows, ensuring earlier detection and treatment. This initiative underscores a broader commitment to curbing STD transmission through proactive, accessible testing solutions.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 12.49 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.6% | 2032 Value Projection: | USD 23.73 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Vela Diagnostics USA Inc., Roche Holdings AG, Alere, Inc., Becton Dickinson & Company, bioMerieux, Danaher Corporation (Beckman Coulter), Hologic, Inc., binx health, Chembio Diagnostics, Pinpoint Science Inc., and bioLytical Laboratories |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The introduction of innovative and user-friendly STD diagnostic kits is significantly contributing to the growth of the global sexually transmitted disease (STD) testing market. A notable example is Invex Health’s announcement on June 20, 2023, about the upcoming launch of Morcheck, India’s first oral HIV self-test kit. Unlike conventional blood-based diagnostics, Morcheck utilizes saliva, making the process non-invasive and more accessible for mass use, especially among individuals hesitant to undergo blood tests. As a third-generation HIV test, it can detect both HIV-1 and HIV-2 antibodies with reliable accuracy. This innovation is a major step in reducing stigma and increasing early detection rates by enabling at-home testing, especially in underserved or rural regions.
By eliminating the need for professional sample collection, such devices streamline the diagnostic process, reduce clinic burden, and encourage proactive sexual health practices. Overall, advancements like Morcheck reflect a shift toward more convenient, accessible, and scalable STD testing solutions worldwide.
Increasing launched of products in the field of STD testing is expected to drive the global diagnostic testing of STDs market growth over the forecast period. For instance, in January 2023, Molbio Diagnostics Pvt. Ltd., an India-based molecular diagnostics company, announced it has launched Truenat HSV 1/2 for testing Herpes Simplex Virus. The test is approved by The Central Drugs Standard Control Organization (CDSCO) and can provide sample-to-test results in an hour. Truenat is a point-of-care portable, battery-operated, IoT-enabled, real-time PCR platform with a sample-to-result time of less than 1 hour. According to the company, the platform can test over 40 diseases, including TB, hepatitis, HIV, HPV, dengue, and malaria. Its rapid turnaround and field applicability make it a vital tool in expanding access to accurate STD diagnostics, especially in low-resource settings. This launch highlights the growing innovation in the sector, a key insight from current diagnostic testing STDs market research, signaling a shift toward rapid, decentralized, and multi-disease diagnostic solutions, further positively influencing the diagnostic testing of STDs market forecast.
Increasing strategies like mergers and acquisitions by key market players is expected to drive the global diagnostic testing of STDs market growth. For instance, on March 15, 2021, F. Hoffmann- La Roche acquired GenMark Diagnostics Inc., a U.S. based molecular diagnostics company, for USD 24.05 per share on a fully diluted basis. GenMark Diagnostics Inc., provides molecular diagnostic tests that are designed to detect multiple pathogens from a single patient sample GenMark’s syndromic panel testing portfolio will complement F. Hoffmann-La Roche current molecular diagnostics portfolio and the F. Hoffmann-La Roche, global network will enable expanded reach for GenMark Diagnostics Inc. products. GenMark Diagnostics, Inc., has proprietary technologies, such as ePlex and eSensor XT-8, that can be utilized by F. Hoffmann-La Roche Ltd. to develop infectious disease tests including bloodstream infections.
The future of diagnostics testing STDs market forecast is poised for significant transformation, driven by technological innovation, growing public awareness, and the increasing demand for accessible, fast, and private testing solutions. One of the key possibilities is the widespread adoption of point-of-care and at-home testing kits offering accurate results without the need for clinical visits. AI-powered diagnostic tools are expected to enhance accuracy and streamline interpretation, while smartphone-integrated biosensors could allow real-time monitoring and reporting.
The market may also witness growth in multiplex testing platforms, which can detect multiple infections (e.g., HIV, syphilis, gonorrhea) in a single test, reducing time and cost. Additionally, blockchain-enabled data systems could improve data privacy and traceability, especially for sensitive health records. Global government collaborations and public-private partnerships are also likely to boost testing reach in underserved regions through mobile labs and subsidized programs.
Together, these developments point toward a future where STD testing is faster, more inclusive, and seamlessly integrated with digital healthcare ecosystems.
The diagnostic testing of STDs market value is no longer just a clinical necessity; it is rapidly becoming a cornerstone of national public health strategy and digital healthcare innovation. However, the market remains deeply fragmented, technically conservative, and hamstrung by systemic underfunding and stigma, despite overwhelming epidemiological urgency.
Moreover, the status quo is unsustainable. The CDC reported over 2.5 million combined cases of chlamydia, gonorrhea, and syphilis in the U.S. in 2023, marking a nearly 20% rise over five years. Yet, test penetration, particularly for asymptomatic patients, is critically low. For example, while chlamydia screening is recommended annually for sexually active women under 25, only ~55% actually undergo testing in the U.S., per the National Committee for Quality Assurance. The gap between infection and detection is not shrinking, it’s widening.
Apart from this, digital self-testing has crossed the reliability threshold but lacks policy traction. Companies like LetsGetChecked, Everlywell, and NOWDiagnostics have released CLIA-certified, FDA-authorized home-based STD test kits that now match lab sensitivity for common pathogens such as Chlamydia trachomatis and Neisseria gonorrhoeae. The First to Know Syphilis Test, launched across U.S. retail channels in 2024, achieved a 96.5% sensitivity in multi-center trials, yet reimbursement frameworks and EMR integration for these tests remain inconsistent.
Definition: Diagnostic testing of STDs is used for the identification and diagnosis of sexually transmitted diseases. These diseases are transmitted through person-to-person sexual contact. The increasing approval and launch of new diagnostic tests are expected to propel the growth of the global diagnostic testing of STDs market over the forecast period. Moreover, increasing incidence of STDs in developing and developed economies along with rising awareness is also expected to drive the market growth during the forecast period.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients