Ebola Vaccine Market Size and Forecast – 2025 – 2032
The Global Ebola Vaccine Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 3.4 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 14.8% from 2025 to 2032.
Global Ebola Vaccine Market Overview
Ebola vaccines are biological products developed to induce immunity against the Ebola virus, which causes severe hemorrhagic fever. These vaccines utilize recombinant viral vector technology, where a harmless virus delivers a gene encoding the Ebola surface glycoprotein to stimulate an immune response. Current vaccines, such as the rVSV-ZEBOV, are administered intramuscularly and provide strong protection against the Zaire strain of the virus. Ongoing developments include multivalent vaccines targeting multiple Ebola species and thermostable formulations suitable for storage and transport in tropical regions.
Key Takeaways
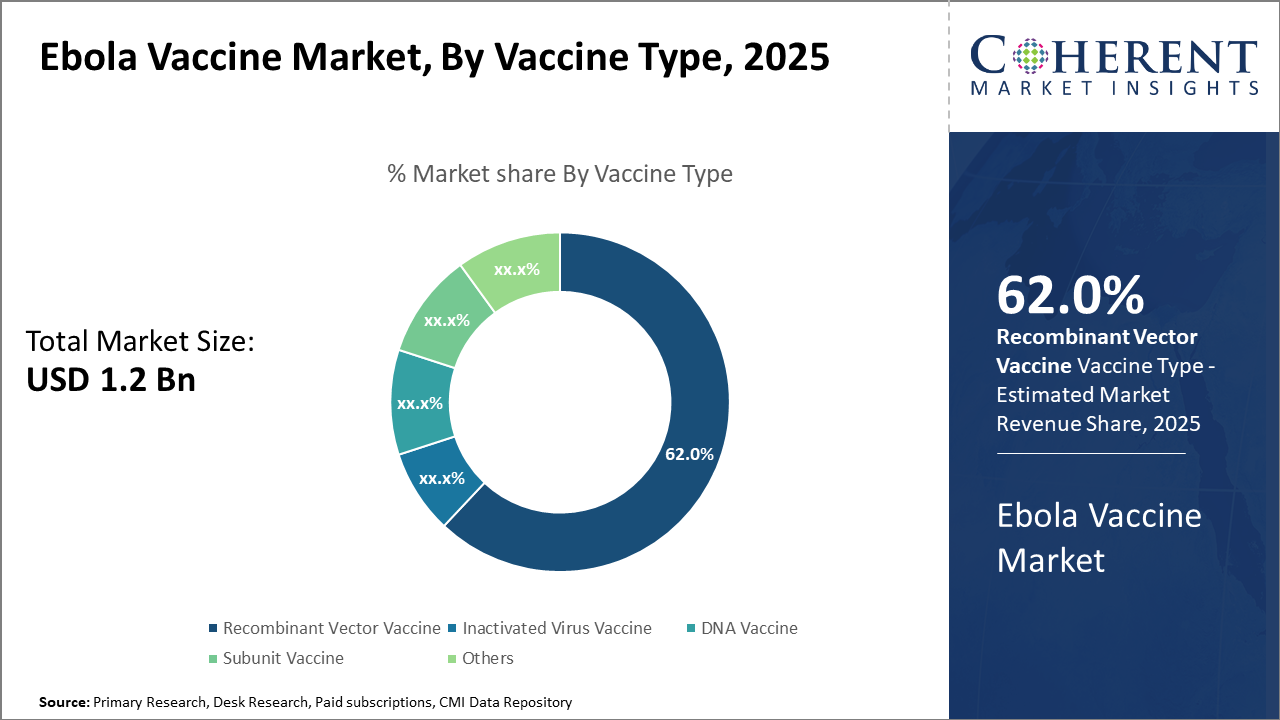
The Recombinant Vector Vaccine dominates the market segment with 62% share, driven by superior efficacy and advanced technology applications.
Government Health Departments lead the end-user segment, reflecting the strategic prioritization of public immunization initiatives.
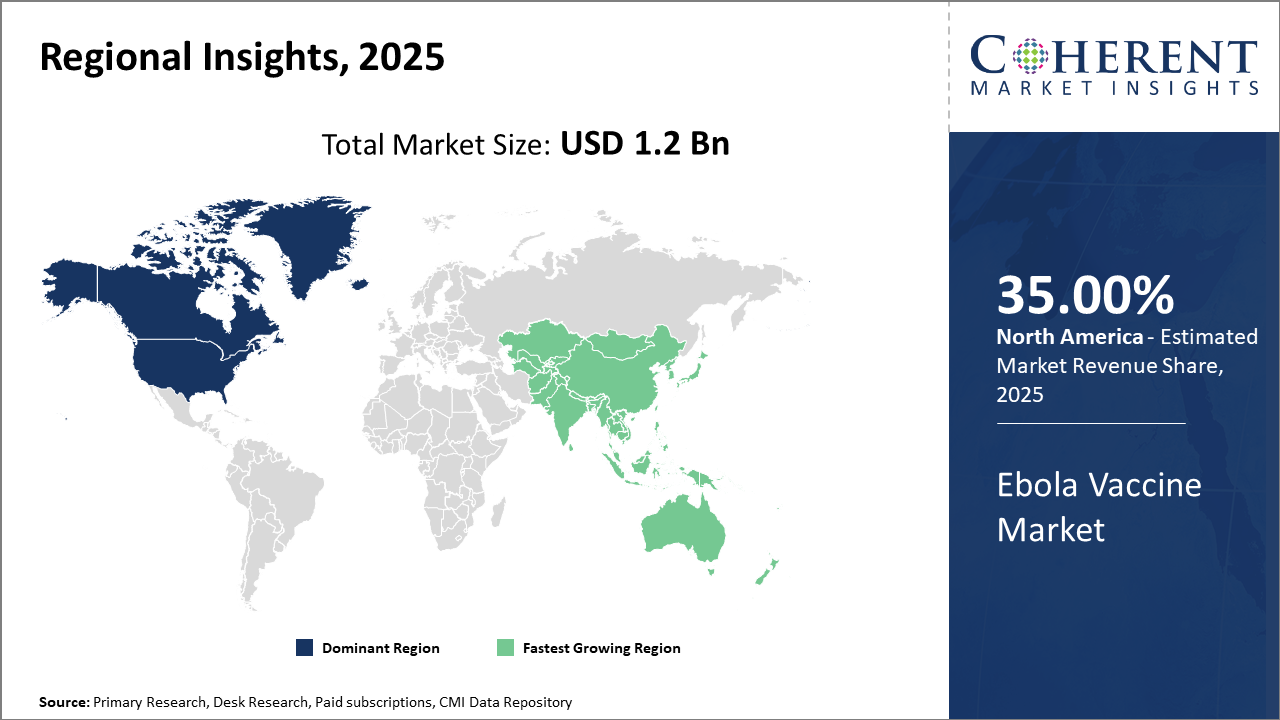
North America maintains dominance in the Ebola Vaccine industry share, contributing a significant portion of market revenue owing to robust R&D infrastructure and regulatory support.
Asia Pacific is the fastest-growing region, exhibiting a CAGR exceeding 16%, bolstered by expanding healthcare frameworks and increasing government vaccination drives in countries like India and China.
Ebola Vaccine Market Segmentation Analysis
To learn more about this report, Download Free Sample
Ebola Vaccine Market Insights, By Vaccine Type
Recombinant Vector Vaccine dominates the market share with 62%, primarily due to its high efficacy and established safety profile demonstrated by Ervebo in controlling outbreaks since 2019. This vaccine type serves as the backbone of many immunization campaigns due to its rapid immune response induction. The fastest-growing subsegment is DNA Vaccine, experiencing accelerated adoption propelled by recent advancements in genetic vaccine technologies showcased in 2024 clinical studies, highlighting promising immune memory.
Ebola Vaccine Market Insights, By End User
Government Health Departments lead this segment, commanding the largest share, fueled by comprehensive vaccination drives and policy-driven procurement. These entities often leverage emergency use authorizations and allocate substantial budgets, as witnessed in the 2024 Ebola outbreak response in West Africa. The fastest-growing end-user subsegment is Research Institutes, spurred by increased funding for vaccine research, epidemiological studies, and clinical trials, fostering ongoing innovation.
Ebola Vaccine Market Insights, By Distribution Channel
Direct Sales remain the dominating subsegment, accounting for a significant market share by enabling direct procurement between manufacturers and large institutional buyers such as governments and hospitals. This channel facilitates timely vaccine availability, critical for emergency outbreak responses. The fastest-growing channel is Online Pharmacies, leveraging digital health infrastructure growth, enabling broader access, particularly in urban and semi-urban locations, with 2024 showing a 22% surge in vaccine sales via this channel.
Ebola Vaccine Market Trends
The Ebola Vaccine market is undergoing a transformative phase with the adoption of cutting-edge vaccine platforms and innovative delivery solutions.
One prominent trend is the rise of recombinant vector vaccines that provide enhanced immunity, as demonstrated by Merck’s Ervebo vaccine achieving a protective efficacy exceeding 90% in outbreak containment in 2024.
Another key trend is the integration of digital cold chain monitoring systems, which have reduced vaccine spoilage rates significantly during transport, especially in remote African regions.
Apart from this, ongoing trials for multivalent vaccines aim to offer broader protection against various Ebola strains and related viruses, addressing shortcomings of monovalent vaccines and providing a new horizon for the vaccine market.
Ebola Vaccine Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Ebola Vaccine Market Analysis and Trends
In North America, the dominance in the Ebola Vaccine market is anchored by advanced research facilities, accelerated regulatory approvals, and substantial government and private-sector investment. The region plays a pivotal role, driven by notable market companies innovating in vaccine platforms and delivery mechanisms, collectively holding over 35% of the global market share. Supportive policies and preparedness programs further consolidate this leadership.
Asia Pacific Ebola Vaccine Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth, with a CAGR surpassing 16%, attributed to burgeoning healthcare investments, government vaccination initiatives, and rising awareness about infectious disease prevention. Emerging markets like India and China are expanding local production capabilities and conducting large-scale immunization campaigns, fostering a dynamic ecosystem for market expansion.
Ebola Vaccine Market Outlook for Key Countries
USA Ebola Vaccine Market Analysis and Trends
The USA’s Ebola Vaccine market is distinguished by high investment in R&D and active participation in global vaccine distribution initiatives. In 2024, the U.S. government allocated an additional USD 400 million towards vaccine stockpiling and research, enabling manufacturers like Merck to expand production capacities by 25%. Collaborative efforts with the CDC have optimized vaccination strategies to support rapid deployment during potential outbreaks worldwide, reinforcing market leadership.
South Korea Ebola Vaccine Market Analysis and Trends
South Korea stands out as the fastest-growing market for Ebola vaccines. This growth is attributed to South Korea's robust healthcare infrastructure, proactive public health strategies, and increasing investments in vaccine research and development. The country's commitment to preparedness against emerging infectious diseases, including Ebola, has contributed to its position as a leader in the Asia-Pacific region's vaccine market.
Analyst Opinion
Increased Production Capacity Accelerating Market Revenue: Production capabilities for Ebola vaccines have expanded significantly post-2023, with several manufacturing facilities in Africa and Europe scaling output to meet global demand. For instance, a leading vaccine producer reported a 35% increase in batch production in H1 2024, supporting wider immunization campaigns. This supply-side growth underpins a robust market revenue uptick and sustained market share enhancement.
Diversified Use Cases Driving Market Share Growth: Beyond outbreak containment, emerging uses of Ebola vaccines in prophylactic healthcare for at-risk populations, such as healthcare workers and travelers, have diversified demand. Controlled vaccination programs in West Africa witnessed a 28% rise in vaccine administration in 2024 compared to previous years, fueling market expansion and contributing to market insights on uptake.
Pricing Dynamics Influencing Market Forecast: Strategic pricing adaptations have been observed, balancing affordability in high-risk zones with profitability in developed countries. Data from late 2024 reveals vaccine pricing becoming 12% more competitive compared to 2023, facilitating wider penetration and favoring positive market growth trends.
Export Volumes Enhancing Industry Size: Export volumes have increased notably via partnerships with global health organizations. For example, export data from 2024 indicates a 40% rise in vaccine shipments to African nations from manufacturing hubs, cementing the industry's share in international markets and enhancing business growth outlook.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 14.8% | 2032 Value Projection: | USD 3.4 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Merck & Co., Johnson & Johnson, Bavarian Nordic A/S, BioProtection Systems Corporation, Pfizer Inc., GlaxoSmithKline plc, Novavax, Inc., Serum Institute of India Pvt. Ltd., Sanofi Pasteur, Valneva SE | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Ebola Vaccine Market Growth Factors
The intensification of Ebola outbreaks across Central and West Africa remains a foremost growth driver, compelling governments and global health bodies to enhance vaccination programs. Increased funding from international health agencies in 2024 contributed more than USD 200 million, stimulating market revenue and expansion. Furthermore, advancements in vaccine technology, particularly recombinant vector platforms showing higher efficacy rates exceeding 90% in recent Phase III trials, are incentivizing accelerated adoption. Heightened regulatory flexibility has also fast-tracked vaccine approval processes, shortening time-to-market and spurring business growth. Moreover, the increasing awareness in endemic and non-endemic regions about preventive immunization and its cost-effectiveness has steadily driven demand, expanding the market scope and influencing favorable market forecasts.
Ebola Vaccine Market Development
In February 2025, Uganda, alongside the World Health Organization (WHO) and the International AIDS Vaccine Initiative (IAVI), launched the TOKEMEZA SVD trial, the first-ever clinical efficacy trial for a vaccine against the Sudan strain of the Ebola virus during an outbreak. The trial employed a ring vaccination strategy to administer IAVI's candidate vaccine to close contacts of confirmed cases, aiming to break chains of transmission and quickly assess the vaccine's effectiveness in the field
In August 2023, the U.S. FDA approved Merck's ERVEBO® vaccine for use in children 12 months and older, establishing it as the first Ebola vaccine available in the U.S. for this age group. The vaccine is a single-dose, intramuscular injection designed to prevent Ebola virus disease (EVD) caused by the Zaire Ebola virus.
Key Players
Leading players in the market include:
Merck & Co.
Johnson & Johnson
BioProtection Systems Corporation
Pfizer Inc.
GlaxoSmithKline plc
Serum Institute of India Pvt. Ltd.
Sanofi Pasteur
Valneva SE
Several market players have adopted strategic collaborations to amplify their reach and impact. For example, Merck & Co. entered a public-private partnership with African governments in 2024 to expedite vaccine distribution, resulting in a 30% increase in administered doses in key regions. Johnson & Johnson’s licensing agreement with African vaccine manufacturers in late 2023 enabled local production, reducing lead times and improving market dynamics in rapidly evolving outbreak zones.
Ebola Vaccine Market Future Outlook
The future of the Ebola vaccine market will revolve around global immunization initiatives, continued public-private collaboration, and advances in multivalent vaccine technology targeting multiple Ebola virus species. Focus will shift toward thermostable, easy-to-administer formulations suitable for tropical field conditions. Emerging R&D pipelines aim to develop combination vaccines addressing related filoviruses. Expansion of stockpiles and integration into routine immunization programs in at-risk regions will ensure preparedness for potential future outbreaks. Strengthened healthcare infrastructure in Africa will further facilitate vaccine access and adoption.
Ebola Vaccine Market Historical Analysis
The Ebola vaccine market evolved rapidly in response to recurring outbreaks in Africa, especially the major 2014–2016 epidemic that underscored the urgent need for preventive measures. Early research focused on vector-based vaccines, leading to the breakthrough approval of rVSV-ZEBOV by regulatory agencies. International partnerships between organizations such as the WHO, Gavi, and leading pharmaceutical firms enabled accelerated development, stockpiling, and deployment in endemic regions. Ongoing surveillance and outbreak preparedness programs sustained demand for vaccine production and distribution infrastructure.
Sources
Primary Research interviews:
Virologists
Infectious Disease Specialists
Vaccine Manufacturing Executives
Public Health Officials
Databases:
World Health Organization (WHO) Vaccine Data
Gavi Vaccine Alliance Database
U.S. FDA Vaccine Approvals
Magazines:
Vaccine Nation
PharmaVoice
Nature Medicine News
Science Daily (Health)
Journals:
The Lancet Infectious Diseases
Journal of Infectious Diseases
Emerging Infectious Diseases
Newspapers:
The Washington Post (Health)
The New York Times (Global Health)
BBC News (Africa)
The Economic Times (Healthcare)
Associations:
World Health Organization (WHO)
Gavi – The Vaccine Alliance
Coalition for Epidemic Preparedness Innovations (CEPI)
Centers for Disease Control and Prevention (CDC)
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients