Medical devices are the electronic products which have medical use and make medical claim. The medical devices includes ultrasound products, x-ray machines, medical lasers and neurological interventional kits. Neuro-interventional devices are catheters-based, minimally-invasive approaches which are used to treat specific diseases of the blood vessels of spine, neck, head and which includes strokes, brain aneurysms, vascular malformation in brain, spine and artery diseases. The several types of Neuro-interventional devices such as flow diverters, stents, and aspiration devices are mainly used for ischemic stroke. There are mainly two types of strokes ischemic and hemorrhagic. Ischemic stroke is common type, and usually cause by blood clotting that blocks blood vessels of brain. Different Neuro-interventional devices like Aspiration device, stenting systems, clot retriever, lasers are used for diagnosis and treatment of ischemic stroke. Procedures like mechanical thrombectomy and carotid endarterectomy are the surgical procedures used to remove a build-up of fatty acid or a thrombus to improve the blood flow through face, neck and brain. As the ischemic stroke are caused by blocking of blood vessels there are several methods to prevent the stroke like controlling high blood pressure, lowering the amount of cholesterol and saturated fat in the food, managing a healthy diet and eating diet rich food like fruits and vegetables. There are different factors which are associated with higher risk of stroke like people of age 55 and above have more risk using hormone therapies can also increase the risk of ischemic stroke.
Global ischemic neurological interventional medical devices market is estimated to be valued at US$ 2,212.7 million in 2022 and is expected to exhibit a CAGR of 5.6% over the forecast period (2022-2030).
Figure 1 Global ischemic neurological interventional medical devices Market Share (%), By End User, 2022
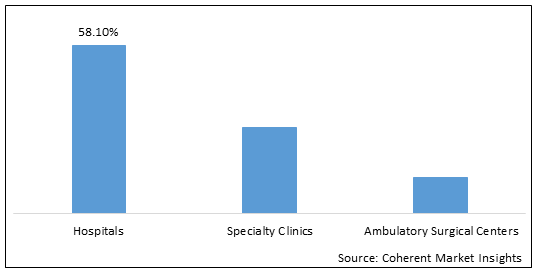
To learn more about this report, Download Free Sample
Global Ischemic Neurological Interventional Medical Device Market- Drivers
The increasing focus on research and development by key players in order to strengthen their market presence in the global market is expected to drive growth of the global ischemic neurological interventional medical devices market over the forecast period. For instance, in October 2020, data was published by Centers for Disease Control and Prevention(CDC), according to which 1 in 6 deaths from cardiovascular disease were due to stroke. Every 40 seconds, someone in the United States has a stroke.
The increasing product launch and product approval by key players in order to strengthen their market presence in the global market is expected to drive growth of the global ischemic neurological interventional medical devices market over the forecast period. For instance, in September 2022, VESALIO, LLC., medical device company, announced the Conformitè Europëenne (CE) Mark approval for their new NeVa NET, micro-filtration technology coupled with NeVa’s Drop Zone technology creates a one-of-a-kind stent retriever, used to capture every type of clot and maximize the retention of clot for fast and effective recanalization of ischemic stroke.
Figure 2. Global ischemic neurological interventional medical devices Market Share (%), By Region, 2022
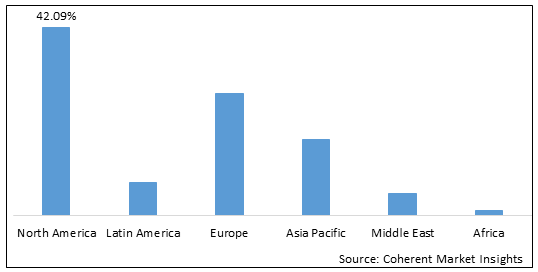
To learn more about this report, Download Free Sample
Global ischemic neurological interventional medical devices Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of medical devices, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of devices from one place to another.
The COVID-19 pandemic and lockdown in various countries across the globe has negatively impacted the financial status of businesses across all sectors. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions.
The COVID-19 pandemic has a negative impact on the neurological interventional medical market. For instance, a paper was published by American Heart Association, Inc. in August, 2021, which suggested that patients with ischemic stroke and COVID 19 infection have more severe strokes while patients with vascular risk factors like diabetes, stroke, obesity, and hypertension are at increased risk of mortality and morbidity by COVID 19.
Ischemic Neurological Interventional Medical Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2022 | Market Size in 2022: | US$ 2,212.7 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 5.6% | 2030 Value Projection: | US$ 3,431.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Stryker, Johnson & Johnson Private Limited., Penumbra, Inc., Medtronic, VESALIO,LLC., Sense Neuro, 880 Medical, LLC., Terumo Corporation., Imperative Care., W. L. Gore & Associate, Inc. , MicroPort Scientific Corporation., KANEKA COPORATION., Integer Holdings Corporation., Wallaby Medical. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Biopharmaceutical and Biomedicine Market: Key Developments
The increasing product launches and approvals are expected to drive the market growth over the forecast period. For instance, in April 2021, VESALIO, LLC ., a company treating stroke and vascular occlusions, initiated a clinical trial to assess the safety, performance and efficacy of NeVa stent retriever a device used to remove the thrombus in patients with ischemic stroke and will be completing the trial in December 2022.
The increasing research and development activities for the development of ischemic neurological interventional medical devices and technologies is expected to drive the market growth over the forecast period. For instance, in January 2022, STROKE: Vascular and Interventional Neurology , an article Published on behalf of the American Heart Association, Inc., and the Society of Vascular and Interventional Neurology by Wiley Periodicals LLC., focused on the paced of advancements and the anticipated trials with the results of acute ischemic stroke.
Global ischemic neurological interventional medical devices Market: Restraint
As there are different trials going on for neurological interventional devices for ischemic stroke, there are more number of premature termination and withdrawal of clinical trials due to failure of not submitting the results within specific deadline, insufficient participation of subjects, device modification, protocol amendments, etc.
For instance, in January 2020, a clinical trial of Thrombectomy device called WOLF, used to remove thrombus, for ischemic stroke was withdrawn due to poor enrollment of subject in the clinical trial. The study was designed to evaluate the safety and device technical performance of the WOLF Thrombectomy Device in removing thrombus or as an adjunct to intravenous tissue plasminogen activator (TPA) and/or to other traditional, thrombectomy modalities.
Global ischemic neurological interventional medical devices Market: Key Players
Major players operating in the global ischemic neurological interventional medical devices market include Stryker, Johnson & Johnson Private Limited., Penumbra, Inc. Medtronic, VESALIO, LLC., Sense Neuro, 880 Medical, LLC., Terumo Corporation., Imperative Care., W. L. Gore & Associate, Inc. , MicroPort Scientific Corporation., KANEKA COPORATION., Integer Holdings Corporation., Wallaby Medical.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients