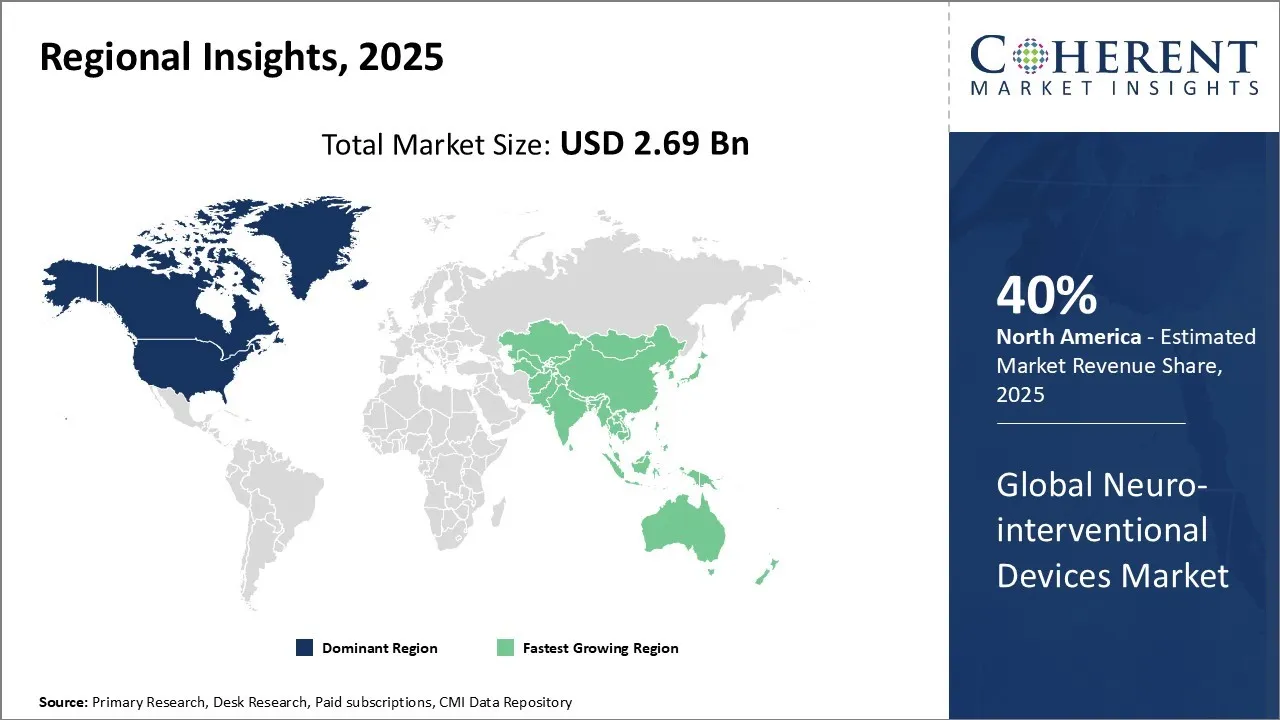
Neuro-interventional Devices Market is estimated to be valued at USD 2.69 Bn in 2025 and is expected to reach USD 3.79 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 5% from 2025 to 2032.
The neuro-interventional devices market demand is growing rapidly due to the rising prevalence of neurovascular disorders such as ischemic stroke, brain aneurysms, and AVMs. Advancements in minimally invasive technologies, including embolic coils, stents, and thrombectomy devices, are improving procedural accuracy and patient outcomes. Coiling procedures remain the most widely adopted, particularly for aneurysm treatment, while ischemic stroke drives the largest application demand.
|
Current Event |
Description and its Impact |
|
Geopolitical and Regulatory Developments |
|
|
Technological Innovations and Clinical Advancements |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement for neuro-interventional devices is a critical factor influencing adoption and hospital investment in these technologies. In the United States, Medicare coverage plays a significant role, although it reimburses only a portion of the costs associated with procedures such as mechanical thrombectomy. Reimbursement for these procedures is typically procedure-based, with specific coding used to reflect the complexity and type of intervention performed. Educational resources from Medicare and device manufacturers provide guidance on coding, coverage, and payment policies, helping hospitals and physicians navigate the reimbursement landscape. In addition, technological advancements, including AI-guided imaging and next-generation catheters, are influencing adoption by improving procedural efficiency and outcomes. The shift toward outpatient and ambulatory surgical center settings is also shaping reimbursement considerations, as cost-effective and shorter-duration procedures become more common. Overall, reimbursement policies remain a key driver in the accessibility and utilization of neuro-interventional devices, directly affecting hospital strategies and patient care delivery.
Artificial intelligence (AI) is playing an increasingly vital role in neurointerventional devices by enhancing imaging, improving procedural precision, and supporting clinical decision-making. AI-powered algorithms enable real-time analysis of complex neurovascular structures, helping clinicians detect abnormalities faster and plan treatments more accurately. In imaging, AI enhances cone-beam CT and angiography by reducing noise and artifacts, resulting in clearer, more detailed views of the brain’s vasculature.
In May 2025, GE HealthCare launched CleaRecon DL, an AI-powered technology designed to enhance cone-beam CT (CBCT) imaging. Recently cleared by the FDA and CE-marked, the deep learning-based solution will be deployed in the U.S. and Europe on the Allia platform. CleaRecon DL improves CBCT image quality by reducing streaks without adding artifacts, supporting clearer visualization for procedures—including neuro.
In terms of end user, the Hospital segment is expected to contribute to highest share of the market in 2025, due to the increasing incidence of neurovascular disorders such as ischemic strokes, brain aneurysms, and arteriovenous malformations (AVMs), which require timely and effective intervention. Neuro-interventional devices, including embolic coils, stents, and thrombectomy systems, enable minimally invasive procedures that reduce patient recovery times, lower complication risks, and improve treatment outcomes. Hospitals are also adopting these devices to enhance procedural precision, expand their neurovascular care capabilities, and meet the growing patient demand for advanced, less invasive treatment options. Additionally, the integration of imaging technologies and navigation systems in hospitals further boosts the neuro-interventional devices market revenue.
For instance, in June 2025, Methodist Hospitals in Northwest Indiana partnered with Philips to enhance its interventional radiology, cardiology, and neurovascular services. The collaboration introduces four advanced interventional labs across the Northlake and Southlake campuses, featuring Philips' Azurion systems equipped with FlexArm and ClarityIQ technology. These state-of-the-art suites aim to improve patient outcomes by enabling minimally invasive procedures such as stroke interventions and complex cardiovascular treatments.
In terms of product type, the embolization coils segment holds a dominant position, holing an estimated hare of 35% in 2025, due to their effectiveness in treating brain aneurysms, arteriovenous malformations (AVMs), and other neurovascular conditions. These coils enable minimally invasive endovascular procedures, avoiding the risks and longer recovery times associated with open brain surgery. They offer precise placement and reliable aneurysm occlusion, improving patient outcomes and procedural success rates. The growing incidence of neurovascular disorders, including ischemic strokes and aneurysms, has further fueled the need for such interventions. Additionally, advancements in coil technology—such as enhanced flexibility, biocompatibility, and deliverability, have increased their efficiency and safety, making embolization coils one of the most sought-after segments in the neuro-interventional devices market share.
For instance, in June 2025, Penumbra, Inc. received FDA clearance for its Ruby® XL System, the largest, longest, and softest coil available for vascular embolization. Designed for large vessel and high-flow embolization, the system aims to enhance procedural efficiency and reduce radiation exposure.
In terms of technique, the coiling procedures segment is expected to lead the market in 2025 with largest share, as they offer a minimally invasive solution for treating brain aneurysms and other vascular abnormalities. Unlike open surgery, coiling involves inserting a catheter into the affected blood vessel and deploying embolic coils to block blood flow to the aneurysm, reducing the risk of rupture. This approach results in shorter recovery times, lower complication rates, and reduced hospital stays, making it safer and more convenient for patients. Additionally, coiling procedures are widely applicable across a variety of aneurysm sizes and locations, increasing their adoption among neurointerventional specialists. Technological advancements in coil materials and delivery systems further enhance procedural precision and efficacy, driving continued neuro interventional devices market demand.
For instance, in January 2025, Boston Scientific completed enrollment in its MAPS™ clinical trial, a prospective, randomized study investigating the efficacy of endovascular coiling for intracranial aneurysms.
To learn more about this report, Download Free Sample
The North America region is projected to lead the market with a 40% share in 2025, due to several key factors. Additionally, the region has a high prevalence of neurovascular disorders, including stroke, aneurysms, and other cerebrovascular diseases, which drives the need for advanced interventional treatments.
Moreover, favorable reimbursement policies and insurance coverage for neuro-interventional procedures make these treatments more accessible to patients. Additionally, the region sees rapid adoption of technological innovations such as AI-assisted imaging, advanced catheters, and minimally invasive devices, which improve procedural outcomes and reduce recovery times.
For instance, in July 2025, Q’Apel Medical announced that its Zebra Neurovascular Access System received U.S. Food and Drug Administration (FDA) clearance. This device is designed for the introduction of interventional devices into the peripheral and neurovasculature. The Zebra system features a unique laser-cut stripe pattern that enhances dynamic flexibility and provides optimal support during device delivery.
The Asia Pacific region is expected to exhibit the fastest growth in the market, due to several key factors. Rising prevalence of neurovascular diseases such as stroke, aneurysms, and arteriovenous malformations is driving the need for advanced treatment options. Increasing awareness among patients and healthcare providers about minimally invasive procedures, combined with growing healthcare infrastructure and investments in advanced medical technologies, is boosting adoption. Government initiatives and public health programs focused on improving neurological care are also supporting neuro interventional devices market growth.
For instance, in May 2025, the Indian government endorsed S3V Vascular Technologies' indigenous thrombectomy device, marking a significant milestone in the nation's medical device innovation. This device is designed to treat acute ischemic strokes by removing blood clots from brain vessels. Developed entirely in India, it aims to reduce dependency on imported medical equipment and enhance accessibility for patients across the country.
The United States is a leading market for neuro-interventional devices, driven by high prevalence of stroke and aneurysms, advanced healthcare infrastructure, and growing adoption of minimally invasive procedures. Reimbursement through Medicare and private insurers, availability of skilled specialists, and technological innovations like AI-assisted imaging and advanced catheters further support demand, making the U.S. a mature and active market for these devices.
For instance, in April 2025, Terumo Neuro announced that its Carotid Stent System has received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA), marking the first dual-layer micromesh carotid stent approved in the United States. This innovative device is indicated for treating carotid artery stenosis in patients at increased risk for adverse events following carotid endarterectomy.
In China, the high incidence of stroke drives strong demand for neuro-interventional procedures. Government investments in advanced neurological care, specialized stroke centers, and modern equipment are improving access to minimally invasive surgery. Growing awareness among healthcare providers and patients about the benefits of early intervention further accelerates the adoption of these devices.
For instance, in August 2025, a recent Chinese multicenter randomized controlled trial (RCT) has demonstrated that the Panvis-A neurointerventional robotic system significantly reduces physician radiation exposure while maintaining procedural performance. Conducted by the Shenzhen Institute of Advanced Biomedical Robot (Abrobo), the study enrolled more than 120 patients undergoing diagnostic cerebral angiography.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.69 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5% | 2032 Value Projection: | USD 3.79 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Penumbra, Inc., Stryker Corporation, Medtronic PLC., Johnson & Johnson Services, Inc., Terumo Corporation, Insera Therapeutics Inc., Anaconda Biomed S.L., NeuroVasc Technologies and Perflow Medical Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The neuro-interventional devices market value is poised to witness transformative advancements driven by rapid technological innovation and an increasing focus on minimally invasive neurological procedures. The growing adoption of next-generation devices, such as flow diverters, thrombectomy systems, and advanced embolization coils, is reshaping treatment paradigms for conditions like ischemic stroke, cerebral aneurysms, and arteriovenous malformations. Notably, studies indicate that mechanical thrombectomy adoption has increased by over 45% in leading healthcare centers across North America and Europe over the past five years, underscoring the shift toward catheter-based interventions.
Furthermore, the precision and efficacy offered by AI-assisted navigation and imaging-integrated platforms are reducing procedure times and enhancing patient outcomes. For instance, hybrid imaging systems combining real-time angiography with 3D reconstruction have demonstrated a 30–40% improvement in procedural success rates for complex intracranial interventions, particularly in anatomically challenging aneurysms.
The competitive landscape is also encouraging rapid innovation. Established medical device companies are strategically partnering with specialized neurovascular startups to accelerate R&D pipelines. Examples include collaborations between Medtronic and specialized microcatheter developers, which have facilitated the introduction of devices capable of navigating tortuous vascular anatomy with higher safety margins.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients