Pediatric Palliative Care Drugs Market is estimated to be valued at USD 91.79 Bn in 2025 and is expected to reach USD 130.02 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.1% from 2025 to 2032.
Analysts’ Views on the Global Pediatric Palliative Care Drugs Market:
Increasing prevalence of chronic diseases, new product launches, and strategies like mergers, acquisitions, and collaboration are expected to drive the global pediatric palliative care drugs market growth over the forecast period. For instance, according to the data published by Centers for Disease Control and Prevention on January 24, 2022, Congenital Heart Defects (CHDs) are the most common types of birth defects. CHDs affect nearly 1% of/or about 40,000 births per year in the U.S. The prevalence (the number of babies born with heart defect compared to the total number of births) of some CHDs, especially mild types, is increasing, while the prevalence of other types has remained stable. The most common type of heart defect is a Ventricular Septal Defect (VSD). About one in four babies with a CHD have a critical CHD. Such diseases require palliative care for neonates. This can drive the growth of global pediatric palliative care drugs market.
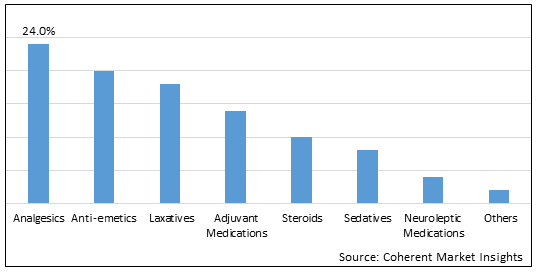
Figure 1. Global Pediatric Palliative Care Drugs Market Share (%), By Drug Class, 2025
To learn more about this report, Download Free Sample
Global Pediatric Palliative Care Drugs Market - Drivers
Increasing launch of newer drugs in pediatric palliative care
Increasing launch of newer drugs in pediatric palliative care are expected to drive the global pediatric palliative care drugs market growth over the forecast period. For instance, on February 15, 2023, Ipilimumab [Yervoy] was approved by the U.S. Food and Drug Administration (FDA) as a single agent or in combination with nivolumab, for the treatment of unresectable or metastatic melanoma in adult and pediatric patients 12 years and older. The approval is assigned to Bristol-Myers Squibb Company, a U.S.-based manufacturer of prescription pharmaceuticals and biologics.
Increasing collaboration strategies by key market players
Increasing collaboration strategies by key market players are expected to drive the global pediatric palliative care drugs market growth. For instance, on March 27, 2023, Teva Pharmaceutical Industries Ltd., an Israel-based global leader in generics and biopharmaceutical medicines, announced a collaboration with Rimidi Inc., a U.S.-based cloud-based software platform that enables personalized management of chronic cardiometabolic conditions across populations for Rimidi’s Respiratory Module for respiratory patient monitoring, based on data collected by Teva’s Digihaler System. Teva Pharmaceutical Industries Ltd. and Rimidi Inc., look to expand respiratory monitoring program to additional health systems with the aim of assessing potential cost savings, hospitalization rates, and the quality of asthma management.
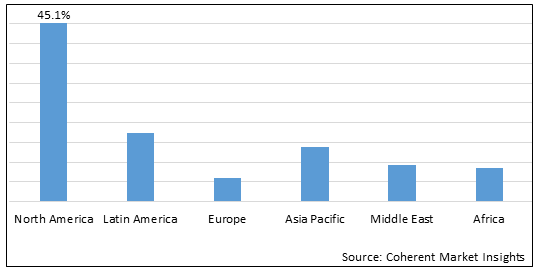
Figure 2. Global Pediatric Palliative Care Drugs Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Pediatric Palliative Care Drugs Market - Regional Analysis
Among region, North America is estimated to hold a dominant position in the global pediatric palliative care drugs market over the forecast period owing to increasing launches of products. For instance, on September 12, 2025, the U.S. FDA announced the approval of Atezolizumab [Tecentriq], as a single agent, for the treatment of adult and pediatric patients 2 years of age and older with unresectable or metastatic alveolar soft part sarcoma (ASPS). The approval was given to Genentech, Inc., a U.S.-based biotechnology corporation.
Global Pediatric Palliative Care Drugs Market - Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global pediatric palliative care drugs market, due to reduced patient visits to hospitals. For instance, according to an article published by Asian Bioethics Review on August 13, 2020, a study was conducted to check the impact of COVID-19 on palliative care. It was found that due to the COVID-19 pandemic, there was interruption in medication supply. The patients who were on medication could not get medicines during the pandemic. Such disruption of supply chains negatively impacted the global pediatric palliative care drugs market.
Pediatric Palliative Care Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 91.79 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.1% | 2032 Value Projection: | USD 130.02 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis International AG, Mylan NV, Teva Pharmaceutical Industries Ltd., Amneal Pharmaceuticals, Inc., Mallinckrodt Pharmaceuticals, Anqiu Lu'an Pharmaceutical Co., Ltd., Granules India Limited, Zhejiang Kangle Pharmaceutical Co., Ltd., Farmson Pharmaceutical Gujarat Pvt. Ltd., Atabay Pharmaceutical Factory, Anhui Fubore Pharmaceutical & Chemical Co., Ltd, Huzhou Konch Pharmaceutical Co., Ltd., Alnylam Pharmaceuticals Inc., Eton Pharmaceuticals, Inc, and Eisai Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Pediatric Palliative Care Drugs Market Segmentation:
The global pediatric palliative care drugs market report is segmented into drug class, route of administration, distribution channel, and region.
Based on drug class, the global pediatric palliative care drugs market is segmented into analgesics, anti-emetics, laxatives, adjuvant medications, steroids, sedatives, neuroleptic medications, and others. Out of which, the analgesics segment is expected to dominate the market due to increasing launch of newer drug products by key market players.
Based on route of administration, the global pediatric palliative care drugs market is segmented into oral, topical, and parenteral. Among these, oral segment is expected to dominate the market over the forecast period due to increased demand for pediatric use.
Based on distribution channel, the global pediatric palliative care drugs market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Among these, hospital pharmacies segment is expected to dominate the market over the forecast period due to increasing numbers of hospital pharmacies.
Based on region, the global pediatric palliative care drugs market is segmented into North America, Europe, Asia Pacific, Latin America, Middle East, and Africa. Among these, North America segment is expected to dominate the market over the forecast period due to increasing launch of newer products in this region.
Among all segmentation, the drug class segment has the highest potential due to increasing launch of new drugs by key market players in this segment. For instance, on November 10, 2022, Brentuximab vedotin [Adcetris] was approved by U.S. FDA for the treatment of pediatric patients 2 years and older with previously untreated high risk classical Hodgkin lymphoma (cHL), in combination with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide. The approval was given to Seagen Inc., a U.S.-based biotechnology company.
Global Pediatric Palliative Care Drugs Market Cross Sectional Analysis:
Among the distribution channel segment, the hospital pharmacies segment is expected to be dominant during the forecast period in Europe due to the increasing launch of newer hospital pharmacies. For instance, on May 27, 2020, European Association of Hospital Pharmacists (EAHP) launched a new COVID-19 resource center for hospital pharmacists. The center was started to assist its member associations and individual hospital pharmacists in this critical time with the provision of the best possible care for patients.
Global Pediatric Palliative Care Drugs Market: Key Developments
On July 14, 2022, the U.S. FDA approved Crizotinib [Xalkori] for the treatment of adult and pediatric patients 1 year of age and older with unresectable, recurrent, or refractory Inflammatory Myofibroblastic Tumor (IMT) that is ALK-positive. The approval is given to PF PRISM CV, a subsidiary of Pfizer Inc., a U.S.-based multinational pharmaceutical and biotechnology corporation.
On August 27, 2020, LHC Group, Inc., a U.S. based health care facilities provider, announced a collaboration with University Health Care System, a U.S.-based healthcare system. The agreement is to form a new joint venture to enhance home health and hospice services across eight cities in Georgia and South Carolina. With this agreement, LHC Group will purchase majority ownership and assume management responsibility of University Health Care System.
On September 23, 2022, European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending a change to the terms of the marketing authorization for the medicinal product crizotinib (Xalkori). The marketing authorization holder for this medicinal product is Pfizer Inc. CHMP adopted two new indications for the treatment of pediatric patients with Anaplastic Large Cell Lymphoma (ALCL) or Inflammatory Myofibroblastic Tumour (IMT).
On August 17, 2022, the government of Canada announced US$ 2 million in support for palliative care for persons who are homeless or vulnerably housed. This funding will allow Healthcare Excellence Canada (HEC), working with partner organizations, such as the Canadian Partnership Against Cancer (CPAC), to help improve the delivery of palliative care services so that people experiencing homelessness or who are vulnerably housed receive safe, timely, appropriate care in the place of their choosing. Hospice palliative care is a critical part of the health care continuum, improving quality of life for as long as possible. Care is provided wherever the person is, be it in a facility or in their community.
Global Pediatric Palliative Care Drugs Market: Key Trends
Funding for better palliative care
Funding for better palliative care can drive the growth of the market. For instance, on December 10, 2021, the Minister for Health, Ireland funded US$ 10.6 million for palliative and end-of-life care through several national organizations, including the Irish Cancer Society. Hence, the new funding for palliative care in cancer is expected to boost the market growth over the forecast period.
Opening of new facilities for pediatric palliative care
Opening of new facilities for pediatric palliative care can drive the growth of the market over the forecast period. For instance, on October 9, 2022, Sparsh Hospice, an India-based hospital, opened a 10-bed pediatric palliative care ward on occasion of World Hospice and Palliative Care Day. These new palliative care facilities increase the widespread services that likely propel the market growth over the forecast period.
Global Pediatric Palliative Care Drugs Market: Restraints
Less research and development in palliative care disease treatment
The less research and development in palliative care disease treatment is expected to hamper the global pediatric palliative care drugs market growth. For instance, according to an article published by Journal JAMA Health Forum on January 28, 2022, the less number of study population is due to ethical issues in pediatric research which reduces pediatric research and development activities. Also poor outcomes, high costs, and persistent inequities for serious medical illness suggests substantial room for improvement.
To counterbalance this restraint, a more research and development using computational models should be carried out.
Lack of awareness about palliative care
In emerging economies, lack of awareness about palliative care is expected to hamper the global pediatric palliative care drugs market growth. For instance, according to an article published in Journal American Journal of Hospice and Palliative Medicine on February 4, 2021, a study was conducted to examine correlates of awareness of palliative, hospice care and advance directives in a racially and ethnically diverse large sample of adults in California, U.S. The study included 2,328 adults. The study concluded that 75% of Hispanics participants claimed that they have never heard about palliative care. Multivariate analysis of data show gender, age, education, and income all significantly were associated with awareness.
To counterbalance this restrain, more awareness programs should be conducted about palliative care.
Global Pediatric Palliative Care Drugs Market - Key Players
The major players operating in the global pediatric palliative care drugs market include Novartis International AG, Mylan NV, Teva Pharmaceutical Industries Ltd., Amneal Pharmaceuticals, Inc., Mallinckrodt Pharmaceuticals, Anqiu Lu'an Pharmaceutical Co., Ltd., Granules India Limited, Zhejiang Kangle Pharmaceutical Co., Ltd., Farmson Pharmaceutical Gujarat Pvt. Ltd., Atabay Pharmaceutical Factory, Anhui Fubore Pharmaceutical & Chemical Co., Ltd, Huzhou Konch Pharmaceutical Co., Ltd., Alnylam Pharmaceuticals Inc., Eton Pharmaceuticals, Inc, and Eisai Inc.
*Definition: Pediatric palliative care is a specialized medical care for infants and children living with serious medical conditions such as cancer, genetic disorders, prematurity, heart and lung conditions, neurologic disorders, and others. The goal of palliative care is to improve the quality of life for both the child and the family by providing relief from the symptoms and stress of the illness.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients