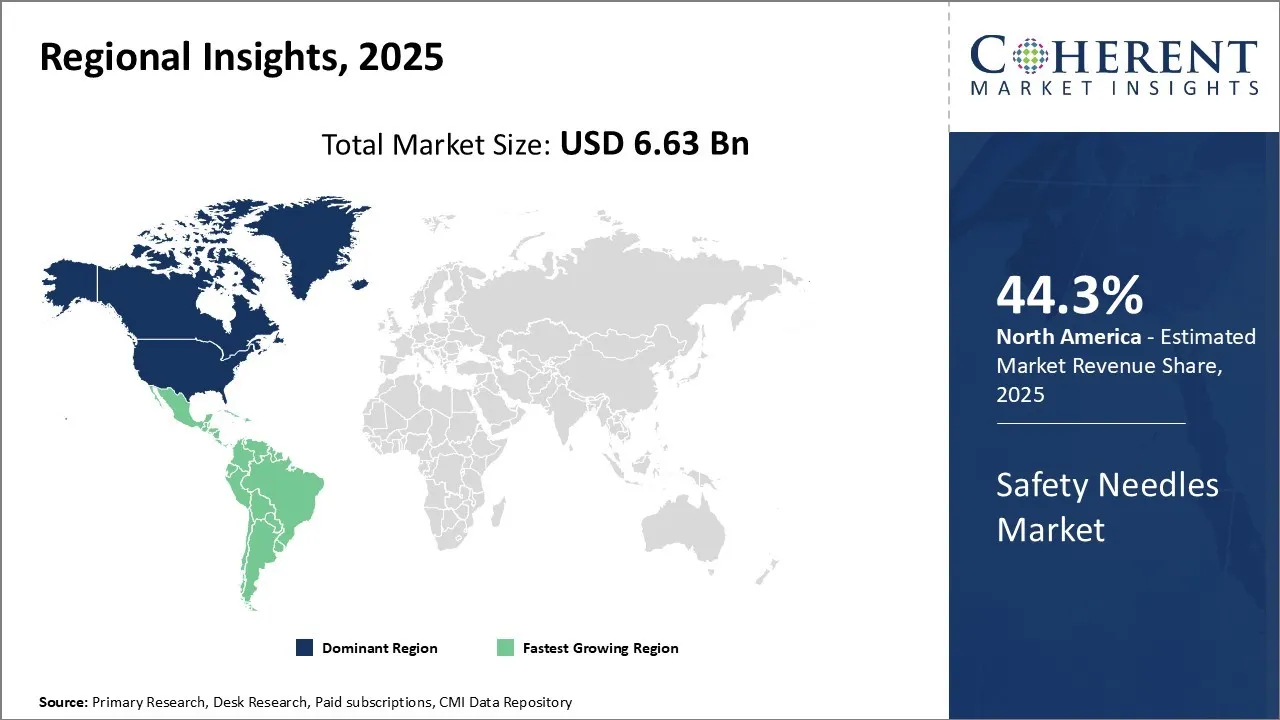
Safety needles market size is estimated to be valued at USD 6.63 Bn in 2025 and is expected to reach USD 11.74 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.5% from 2025 to 2032.
The safety needles market growth is experiencing robust growth, primarily driven by the increasing incidence of needlestick injuries (NSIs) and the consequent risk of transmitting bloodborne diseases like HIV and hepatitis. This has led to heightened awareness and the implementation of stringent regulations promoting the adoption of safety-engineered needles. For instance, the Needlestick Safety and Prevention Act in the U.S. mandates the use of safety needles in healthcare settings. Additionally, the rising prevalence of chronic diseases necessitating frequent injections, such as diabetes and cancer, further propels market demand. In India, government-led vaccination initiatives and efforts to improve injection safety are significantly contributing to market expansion.
|
Event |
Description and Impact |
|
Increasing Prevalence of Chronic Diseases |
|
|
Technological Advancements in Safety Needles |
|
|
Stringent Government Regulations and Safety Standards |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The hypodermic needle segment is expected to dominate the safety needle market forecast owing to large volume of hypodermic needle market share of 17.0% that are being used in healthcare industries for diverse medical conditions.
The drug delivery segment is expected to hold a dominant position in the safety needles market during the forecast period and this is attributed to the increasing usage in growing clinical applications.
The hospital segment is expected to dominate the market over the forecast period and this is attributed to the increasing prevalence of chronic diseases such as HIV, cancer therapy and spine disorders.
To learn more about this report, Download Free Sample
North America is estimated to hold a dominant position in the global safety needles market trend over the forecast period. North America is estimated to hold 44.3% of the market share in 2025. The growing prevalence of chronic diseases and spine disorders directly impacts the increased demand for the safety needles market.
For instance, in July 2022, according to an article published by StatPearls publishing, in North America, millions of healthcare workers use needles in their daily work, and hence, the risk of needlestick injuries is always a concern. While the introduction of universal precautions and safety conscious needle designs has led to a decline in needlestick injuries. However, the major concern after a needlestick injury is not HIV but hepatitis B or hepatitis C.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 6.63 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.5% | 2032 Value Projection: | USD 11.74 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Plc. Becton Dickinson Company, Boston Scientific Corporation, Smith Medical, Inc., Abbott Laboratories, Argon Medical Devices, Inc., Novo Nordisk A/S, Terumo Corporation, Eli Lily and Company, Nipro Medical Corporation, and B. Braun Melsungen AG. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
To prevent the needlesticks injuries, the needs of safety needles conducting both prenatal and postnatal research, key market players are focused on launching new products to expand their product portfolio.
For instance, on January 10 2023, Sharps Technology, Inc., an medical device and drug delivery company offering patented, best-in-class syringe products, announced the advancement of the company's specialized prefillable syringe ("PFS") system product line, which will be manufactured in collaboration with Nephron Pharmaceuticals at the Inject EZ facility in West Columbia, South Carolina, U.S.
Rising incidence of chronic diseases like HIV, cancer, hepatitis and spine disorders in emerging economies is also expected to boost demand for safety needles. For instance, on January 25 KORU Medical Systems, Inc., a medical technology company focused on the development, manufacturing, and commercialization of innovative and easy-to-use subcutaneous drug delivery device that improve quality of life for patients, announced a development agreement with a pharmaceutical manufacturer of subcutaneous immunoglobulin therapy (SCIg) to develop and seek regulatory approval of the Freedom Infusion System with an SCIg prefilled syringe.
Due to improved healthcare infrastructure, effective government policies, and a sizable base of multinational corporations, the safety needles market is expected to experience considerable expansion in the North American region. Products with advanced features such as self-retracting needles and needles with safety locking system are gaining traction among healthcare professionals and patients.
In October 2021, West Pharmaceutical Services, a designer and manufacturer of injectable pharmaceutical packaging and delivery systems announced its latest offering for the Indian domestic market – NovaGuard SA Pro Safety System, a single-use accessory for prefilled ISO standard 0.5mL staked needle syringes.
Effective government policy such as awareness about the proper usage of needles, running campaigns for the safety measures, and so on is expected to drive the global safety needle market. For instance, in April 2022, American Medical Association (AMA) urges states to adopt new Maine needle, syringe exchange policy to modify restrictive laws and regulations concerning the sale and possession of needles and syringes to maximize the availability of sterile syringes and needle.
Major players operating in the global safety needles market include Medtronic, Plc. Becton Dickinson Company, Boston Scientific Corporation, Smith Medical, Inc., Abbott Laboratories, Argon Medical Devices, Inc., Novo Nordisk A/S, Terumo Corporation, Eli Lily and Company, Nipro Medical Corporation, and B. Braun Melsungen AG.
*Definition: - Safety needles are created to stop needlestick wounds and lessen the chance of infection and contamination in medical environments. They exist in several varieties, such as blunt-tip, sheathed, and retractable needles. The prevalence of needlestick injuries, which can result in the spread of bloodborne infections like hepatitis B, hepatitis C, and HIV, has been demonstrated to decrease with the use of safety needles. The rise in chronic diseases such as cancer, HIV, and spine problems, as well as the rising older population, are both credited with driving the market's expansion. Additionally, the increasing awareness about the risks associated with needlestick injuries and the need for safe injection practices is also expected to contribute to the growth of the safety needle market.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients