Sterile Injectables Market is estimated to be valued at USD 632.46 Bn in 2025 and is expected to reach USD 1,077.62 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.9% from 2025 to 2032.
To learn more about this report, Download Free Sample
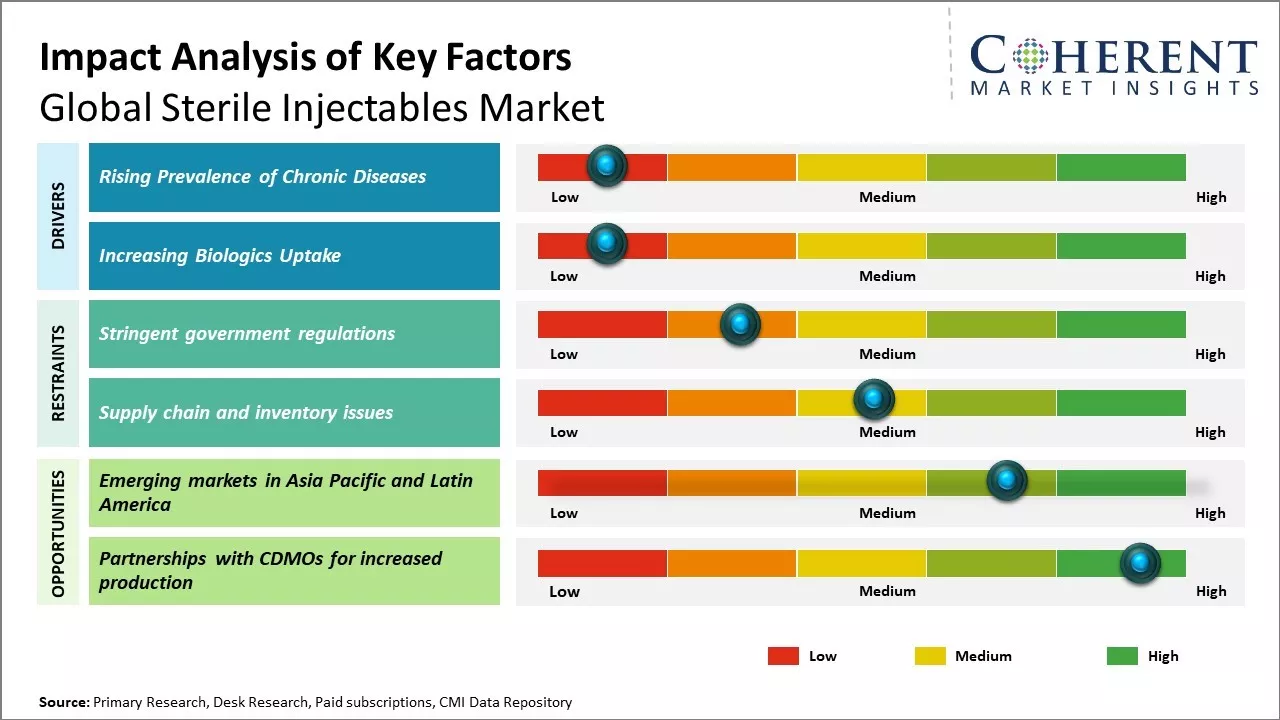
The Sterile Injectables Market Size is expanding rapidly, with forecasts hitting and the sectors growth being supported by improvements in drug research, a solid pipeline of new treatments, and rising demand for both novel injectables and generics. Global sterile injectables market growth is driven by rising prevalence of chronic diseases worldwide. Furthermore, rising geriatric population who are more prone to develop chronic disease conditions can also boost demand for sterile injectables.
For instance, in January 2025, Akums Drugs and Pharmaceuticals has launched a new sterile facility designed to produce lyophilised products, injectables, vials, ampoules, eye and ear drops, and form-fill-seal (FFS) products.
|
Event |
Description and Impact |
|
Surge in Biologics and Biosimilars Adoption |
|
|
Expansion of Manufacturing Facilities in Europe |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The sterile injectables pipeline is robust, with pharmaceutical companies prioritizing pilot-scale fill-finish facilities to support clinical development and commercialization. The rise of biologics and biosimilars is fueling the pipeline, particularly in oncology, vaccines, and chronic disease therapies. Notably, over 200 antibody-drug conjugates (ADCs) are in clinical trials, and contract development and manufacturing organizations (CDMOs) are expanding capacity to meet this demand. Investments in new aseptic filling lines and modernized manufacturing facilities are aimed at alleviating drug shortages and supporting both novel and generic injectables.
The patent landscape for sterile injectables is dynamic, with innovation concentrated in biologics, delivery systems (such as prefilled syringes and cartridges), and aseptic processing technologies. Patent expiries for key biologics are driving the entry of biosimilars, intensifying competition and encouraging further R&D investment. Companies are also patenting advances in AI-enabled quality control and automated fill-finish technologies to secure competitive advantages in manufacturing efficiency and compliance.
In terms of molecule type, large molecules segment is expected to contribute the highest market share due to rising adoption of biologics for treatment. Biologics, such as monoclonal antibodies, have revolutionized the treatment of various chronic and life-threatening diseases. Conditions like rheumatoid arthritis, psoriasis, and cancers that were previously difficult to treat can now be managed more effectively with biologics.
Furthermore, development of sophisticated recombinant DNA technology has enabled the production of complex biologic molecules on a commercial scale. This has significantly boosted research and development of novel biologic drugs for a wide range of indications. Due to their high specificity and precision in targeting molecular disease pathways, there has been increased use of biologics.
Unlike traditional small molecule drugs, biologics interact with cellular antigens or receptors with pinpoint accuracy. This translates to superior clinical efficacy along with lesser side effects as compared to non-targeted therapies.
Advancements in protein engineering and manufacturing techniques have enhanced biologics' drug-like properties such as extended half-life and convenient dosing regimens. These developments have broadened biologics' applications and encouraged their adoption for long-term disease management. The patent expiries of blockbuster biologics have also spurred product line extensions through biosimilars, thus, driving the segment growth.
In terms of drug type, monoclonal antibodies segment is expected to contribute the highest market share of, owing to its diverse therapeutic applications. Monoclonal antibodies are biologic drugs derived from living cells in a laboratory to act against specific antigens or receptors involved in disease pathways. Their exquisite molecular specificity and high affinity binding translate to robust clinical responses.
With advancement in genetic engineering and hybridoma technology, scientists are now capable of developing monoclonal antibodies against a wide array of disease targets. This has translated to monoclonal antibodies emerging as a highly effective treatment option for various cancers, inflammatory disorders, cardiovascular diseases and more.
As research continues to illuminate novel pathogenic targets, the therapeutic scope of monoclonal antibodies will keep expanding to new disease areas. Their versatile structure also allows for drug modifications like antibody-drug conjugates and bispecific antibodies with improved pharmacological properties. Continuous innovation and increasing understanding of disease biology can drive the monoclonal antibodies segment growth over the forecast period.
In terms of disease indication, cancer segment is expected to contribute the highest market share of owing to growing disease prevalence globally. According to WHO, cancer incidence is rising steeply worldwide with over 19 million new cases reported in 2021. As economies develop and life expectancy increases in developing nations, cancer cases are projected to increase, thus, placing a huge disease burden. This upsurge in cancer patients boosts demand for effective oncology therapies.
Sterile injectable drugs are integral to modern cancer treatment in the forms of monoclonal antibody drugs, cytotoxic chemotherapeutics and supportive care agents. Their administration through the parenteral route enables optimal drug bioavailability to exert immediate therapeutic effects.
Furthermore, injectable drugs remain irreplaceable for targeted delivery of anticancer payloads to tumors via technologies like antibody-drug conjugates. With continuous progress in elucidating tumorigenesis pathways, novel injectable drugs are transforming cancer management. Sustained research also aims to enhance the precision and tolerability of oncology injectables.
In terms of route of administration, Intravenous (IV) segment is expected to contribute the highest market share of owing to growing disease prevalence globally. The intravenous (IV) segment is a key route of administration within the sterile injectables market, playing a significant role in the delivery of a wide range of pharmaceutical products.
Sterile injectable drugs are typically administered intravenously, as this method allows for rapid onset of action, precise dosing, and is particularly effective for treating acute and chronic conditions such as cancer, cardiovascular diseases, and infectious disorders. The IV route is favored in clinical settings due to its ability to deliver medications directly into the bloodstream, ensuring immediate therapeutic effects and high bioavailability.
To learn more about this report, Download Free Sample
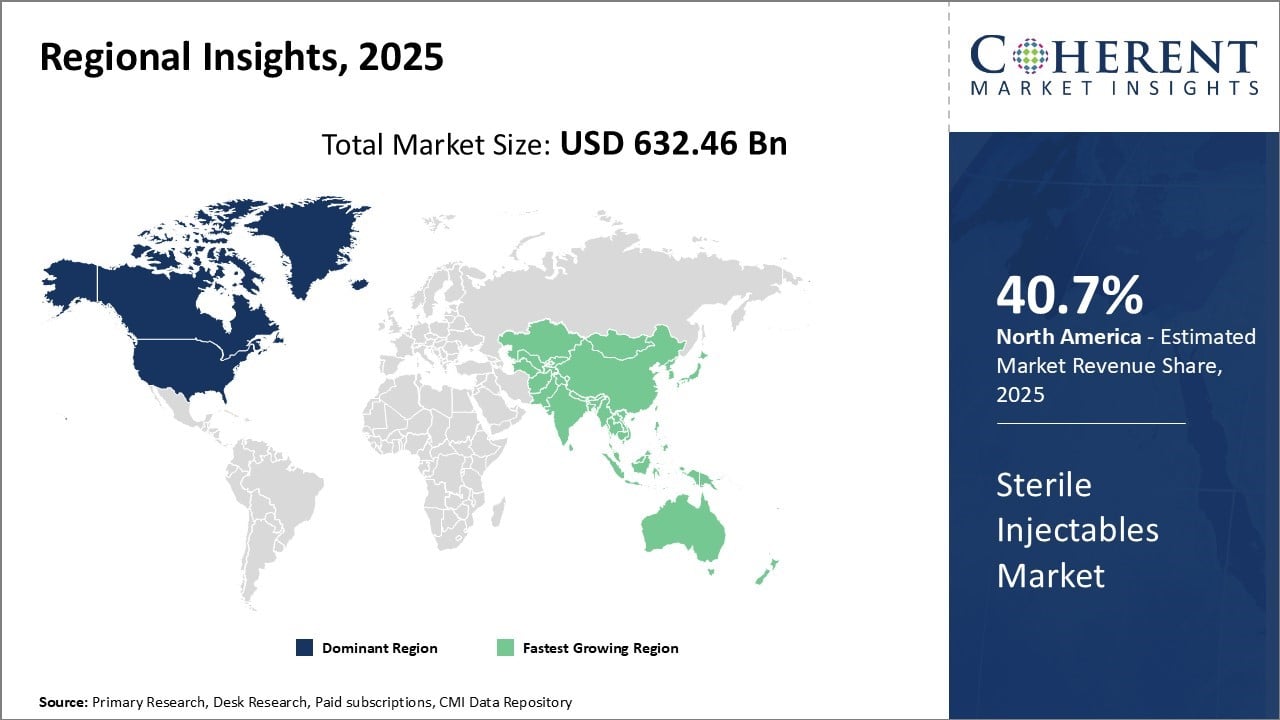
North America dominates the global sterile injectables market with an estimated market share of 40.7% in 2025, due to strong presence of major pharmaceutical companies in the region. Countries like the U.S. have highly developed healthcare systems rapidly adopts new technologies. The region is home to numerous contract manufacturing organizations that cater to the sterile injectables production needs of pharmaceutical firms.
This has ensured strong production capacities are available within the region to cater to domestic demand. Furthermore, presence of several pharmaceutical giants with large R&D capabilities has resulted in many patent-protected drugs being manufactured in North America first before being supplied globally. This first-mover advantage allows companies to capture higher prices and market share for new drug launches in this region.
Asia Pacific region has emerged as the fastest growing market for sterile injectables. Countries like China, India and South Korea have witnessed explosive growth in their pharmaceutical industries. Extensive efforts by their governments to promote domestic manufacturing has boosted production capacities in these nations.
For instance, China offers tax incentives and Special Economic Zones for companies setting up or expanding sterile injectable manufacturing sites. This attracts significant investments from global pharmaceutical companies to cater to the Asian patient pool. India has also liberalized its regulations for imports and exports while protecting intellectual property, making it an attractive alternative manufacturing destination.
The comparatively lower costs of operations ensure companies can supply these emerging Asian markets, as well as export to other regions, in a very cost-effective manner. Availability of a large talent pool of skilled life sciences professionals further supports their rise as global sterile injectables hubs.
Europe benefits from a well-established healthcare infrastructure, high per capita healthcare spending, and the presence of major pharmaceutical and contract development and manufacturing organizations (CDMOs), which are increasingly adopting artificial intelligence and automation to enhance efficiency and compliance.
The United States is the dominant force in the global sterile injectables market, driven by a high prevalence of chronic diseases such as cancer and diabetes, as well as significant advancements in biotechnology and biologics.
India is emerging as a key player in the global sterile injectables market, leveraging its large pharmaceutical manufacturing base and cost-effective production capabilities. The country is a major supplier of generic sterile injectables, meeting both domestic and international demand.
China is rapidly expanding its footprint in the sterile injectables market, supported by strong government initiatives to modernize healthcare, a large patient population, and significant investments in pharmaceutical R&D.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 632.46 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.9% | 2032 Value Projection: | USD 1,077.62 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Merck & Co., Inc., Johnson & Johnson, Baxter International Inc., Novartis AG , Sanofi S.A., GlaxoSmithKline plc (GSK), AstraZeneca PLC, Gilead Sciences, Inc., Amgen Inc., AbbVie Inc., Teva Pharmaceutical Industries Ltd., Bayer AG, Fresenius SE & Co. KGaA (Fresenius Kabi), Becton, Dickinson and Company (BD), F. Hoffmann-La Roche Ltd and Eli Lilly and Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Rising prevalence of chronic diseases such as cancer, diabetes and cardiovascular diseases can drive the sterile injectables market growth. These chronic diseases usually require long term treatment and sterile injectables play a vital role in providing effective treatment to patients suffering from such conditions. Sterile injectables are widely used in delivering drugs for cancer treatment intravenously or subcutaneously.
Many chemotherapy drugs that are used to destroy cancer cells are available only in injectable form. According to studies, there will be increase in cancer cases in the near future due to aging population and lifestyle changes across both developed and developing nations. This can boost demand for various oncology sterile injectable drugs. Sterile injectables have emerged as an indispensable mode of administration for treatment of diabetes. Insulin is used in the form of sterile injectable for diabetes patients.
Growing prevalence of diabetes can boost demand for insulin sterile injectables. Increasing obesity levels and lack of physical activity can add more patients to the diabetes population pool necessitating glucose management via additional insulin doses.
For instance, in May 2022, according to the article published by BioMed Central Journal, 26.7 million Indians were estimated to be cancer patients, and by 2025, that figure is expected to rise to 29.8 million. IDF reported that approximately 3.9 million French citizens had diabetes in 2022; and by 2030, that figure is expected to rise to 4.1 million, and by 2045, it is predicted to reach 4.2 million. Therefore, it is projected that rising prevalence of chronic illnesses can boost demand for safe and efficient injectable medications.
Rising adoption of biologics like monoclonal antibodies, hormones and vaccines can drive the global sterile injectables market growth. Biologics have revolutionized the treatment of various severe medical conditions including rheumatoid arthritis, psoriasis, cancer and others due to their high specificity and efficacy. Their administration method relies heavily on sterile injectable delivery routes like intravenous or subcutaneous injections. Sterile injectables allow for sustained release of complex biologic molecules into the body over an extended period to achieve the desired therapeutic effect. Injectable biologics face less gastric acid destruction and enzymatic degradation issues compared to oral administration paths. These advantages have made sterile injectables the preferred formulation for biologics.
The sterile injectable market is experiencing strong growth, shaped by several key trends. Rising prevalence of chronic diseases and an aging global population are major drivers, increasing the demand for injectable therapies, particularly for conditions like cancer, diabetes, and cardiovascular diseases.
There is a notable shift toward biologics and large molecule drugs, with monoclonal antibodies and advanced therapies fueling market expansion and accounting for a significant share of new product launches. The adoption of ready-to-use injectable systems such as prefilled syringes and autoinjectors is accelerating, driven by their safety, convenience, and ability to support self-administration, especially in home care and chronic disease management.
Emerging markets in Asia Pacific and Latin America can offer significant potential opportunities for global sterile injectables market growth. These regions have large populations and growing healthcare needs that boosts demand for sterile medical products and treatments.
Key developing countries in these regions like India, China, Brazil and Mexico have been witnessing steady economic expansion, and this has improved access to healthcare for many citizens. This, coupled with rising incomes, has increased the ability of patients to afford important sterile injectable medicines for chronic conditions.
Governments in emerging nations have started prioritizing healthcare and aims to provide universal access to services. For instance, in 2022, according to the report published by World Bank, India's total healthcare spending as a percentage of GDP had increased from 1.2% in 2014 to 3.5% in 2021. This expanded coverage and support from the government allows more patients to avail sterile injectable drugs and therapies.
Asia Pacific region has witnessed rising cases of non-communicable diseases like cancer, diabetes and cardiovascular issues linked to changing lifestyles and aging populations. According to the data from the International Diabetes Federation's Diabetes Atlas, in 2021, over 150 million adults in the region have diabetes with China and India accounting for the most cases globally. Treating such conditions generally involves regular injections and intravenous therapies. Thus, growing disease burden boosts demand for sterile injectable pharmaceutical products.
*Definition: Global Sterile Injectables Market refers to the market for sterile injectable pharmaceutical formulations that are administered parenterally into a patient's body in a sterile manner. Sterile injectables include vials or ampoules of sterile liquid solutions/suspensions, prefilled syringes, and ready-to-use small volume parenteral. The sterile injectable drugs are free from microorganisms and particulate matter to maintain sterility from the point of manufacturing until administration to patients. Common sterile injectable products include vaccines, monoclonal antibodies, hormones, anti-infectives, and cytostatic agents.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients