Tardive Dyskinesia Drugs Market Size and Forecast – 2026 – 2033
The Global Tardive Dyskinesia Drugs Market size is estimated to be valued at USD 2.30 billion in 2026 and is expected to reach USD 4.80 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 11.2% from 2026 to 2033.
Global Tardive Dyskinesia Drugs Market Overview
Tardive dyskinesia (TD) drugs are medications used to manage the involuntary, repetitive movements caused by long-term use of certain antipsychotic or neurological drugs. The most commonly prescribed TD treatments are VMAT2 inhibitors, such as valbenazine and deutetrabenazine, which work by regulating dopamine levels in the brain. These drugs help reduce abnormal movements affecting the face, tongue, lips, or limbs. In some cases, doctors may adjust or change the original antipsychotic medication. TD drugs do not cure the condition but can significantly improve symptoms and quality of life when taken under medical supervision.
Key Takeaways
The VMAT2 inhibitors subsegment leads the market, driven by superior efficacy and strong clinical validation.
Adult Patients is the largest segment, due to high prevalence and drug-induced movement disorders.
Hospital pharmacies are the preferred distribution channel, supported by institutional prescription protocols and immediate patient access.
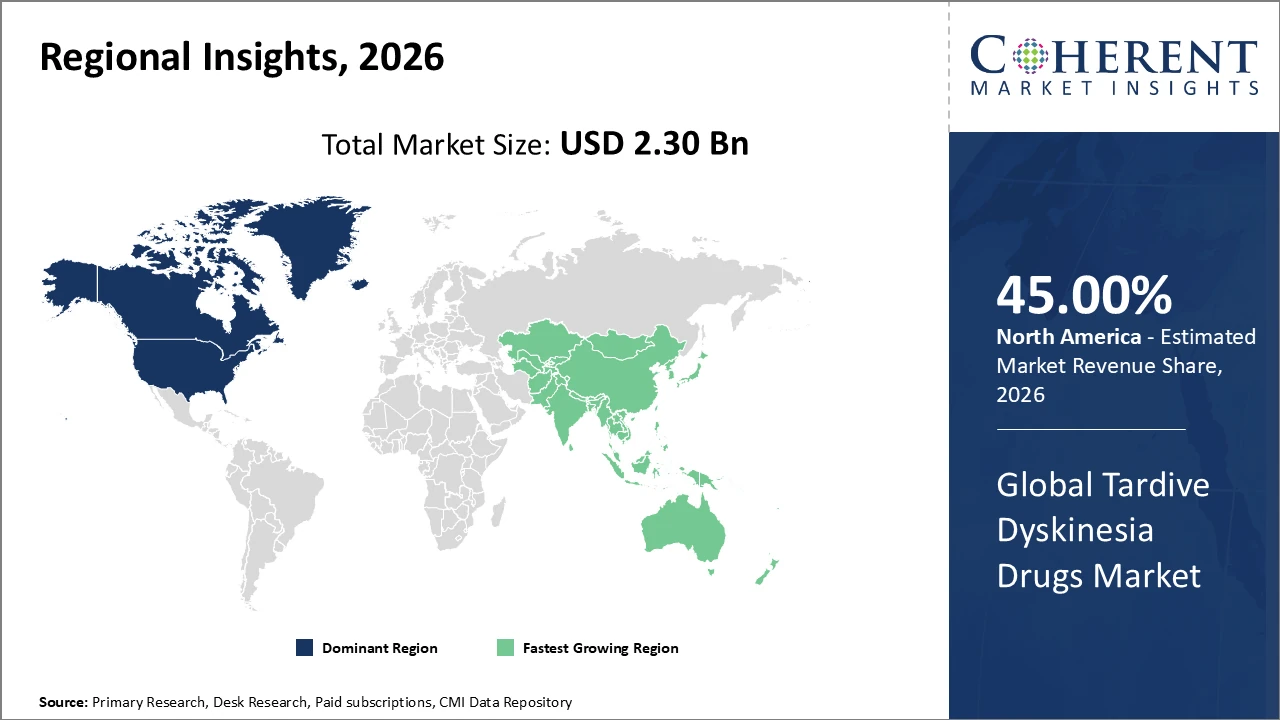
North America dominates the market with over 45% industry share, owing to advanced healthcare infrastructure and higher awareness.
Asia Pacific is the fastest-growing region, with a CAGR exceeding 10%, driven by expanding healthcare access, rising diagnosis rates in India and China, government initiatives, and increased healthcare investment.
Tardive Dyskinesia Drugs Market Segmentation Analysis
To learn more about this report, Download Free Sample
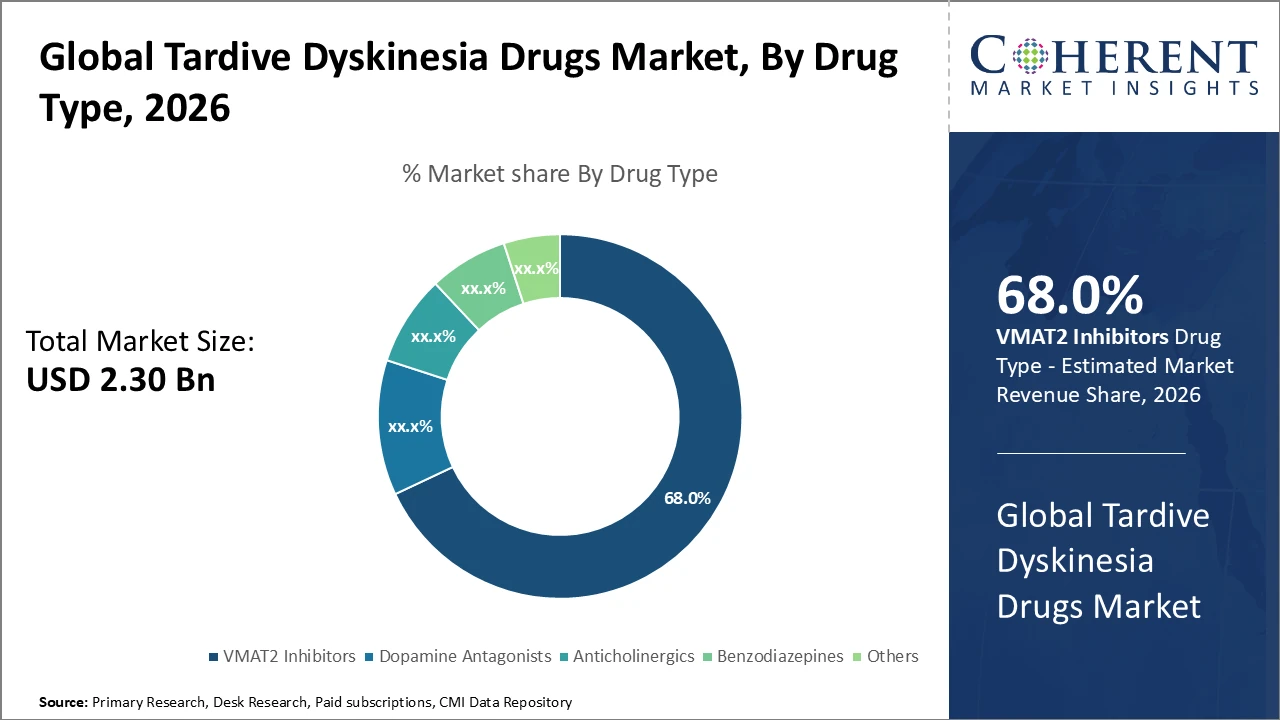
Tardive Dyskinesia Drugs Market Insights, By Drug Type
VMAT2 inhibitors dominate the market with a 68% share, driven by clinical validation demonstrating superior efficacy in reducing involuntary movements characteristic of tardive dyskinesia. The fast-growing subsegment, anticholinergics, has gained renewed interest, especially in emerging regions, due to lower cost and greater accessibility. Anticholinergics help mitigate symptoms but have a distinct side effect profile. Benzodiazepines and other therapies serve as adjunct or alternative options for niche patient populations. Overall, the drug type segment is shaped by clinical efficacy, patient tolerability, and ongoing research into novel mechanisms of action.
Tardive Dyskinesia Drugs Market Insights, By Application
The adult patients segment dominates the market due to the high prevalence of tardive dyskinesia (TD) secondary to antipsychotic use in this population. This segment benefits from widespread drug availability and well-established clinical guidelines. Notably, the geriatric patients subsegment is the fastest-growing, driven by aging populations worldwide and increased susceptibility to neuroleptic-induced movement disorders. Recent demographic studies from 2026 indicate an 18% increase in TD diagnoses among elderly patients. The pediatric patients subsegment remains niche, primarily limited by safety concerns. The others category includes rare off-label and research indications.
Tardive Dyskinesia Drugs Market Insights, By End-User
Hospital pharmacies dominate the market, driven by the institutional nature of TD diagnosis and treatment initiation, allowing close monitoring of adverse effects and adherence to treatment protocols. Online pharmacies are the fastest-growing channel, supported by patient convenience, digital health adoption, and telemedicine integration. Retail pharmacies maintain substantial market share, particularly in regions with strong outpatient care infrastructure. The “Others” category includes specialized clinics and compounding pharmacies, catering to customized treatment needs. Overall, the distribution channel landscape is evolving alongside broader healthcare delivery transformations, reflecting shifting patient preferences and advances in technology.
Tardive Dyskinesia Drugs Market Trends
Increased adoption of VMAT2 inhibitors is driving market trends, revolutionizing treatment with superior safety and efficacy profiles.
Real-world data from North American neurological centers in 2025 show a 20% increase in patient adherence, boosting market revenue.
Integration of digital technology with pharmaceuticals enables real-time symptom tracking and personalized therapy adjustments, increasingly adopted in developed markets.
Regulatory frameworks have shifted to expedite approval of breakthrough treatments, with multiple fast-track approvals granted in 2024 and 2025 in the U.S. and Europe.
These trends reinforce speed-to-market strategies for key companies in the TD drugs market.
Tardive Dyskinesia Drugs Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Tardive Dyskinesia Drugs Market Analysis and Trends
North America dominates the Tardive Dyskinesia Drugs Market, accounting for approximately 45% of total revenue, driven by a mature healthcare ecosystem and well-established neurology infrastructure. The presence of leading pharmaceutical companies, such as Neurocrine Biosciences and Teva, has strengthened market development through innovative therapies and strategic launches. High patient awareness, coupled with strong government reimbursement policies, facilitates widespread adoption of VMAT2 inhibitors and other TD treatments. Additionally, advanced clinical research, real-world evidence studies, and digital health integration support improved patient adherence and outcomes. These factors collectively reinforce North America’s leading position and sustained market growth in the global TD drugs landscape.
Asia Pacific Tardive Dyskinesia Drugs Market Analysis and Trends
Asia Pacific is the fastest-growing region in the Tardive Dyskinesia Drugs Market, with a CAGR exceeding 10%, driven by rising geriatric populations and increasing mental health awareness campaigns across India, China, and Southeast Asia. Expanding healthcare infrastructure, including specialized neurology centers and improved diagnostic capabilities, supports wider patient access and treatment adoption. The emergence of pharmaceutical manufacturing hubs in countries like India and China enhances local production and cost-effective distribution. Strategic entry and investment by global market players further accelerate their presence in the region, fostering competitive growth. These factors collectively position Asia Pacific as a key driver of global TD market expansion.
Tardive Dyskinesia Drugs Market Outlook for Key Countries
USA Tardive Dyskinesia Drugs Market Analysis and Trends
The USA is the largest single-country market for Tardive Dyskinesia drugs, driven by high schizophrenia prevalence and widespread adoption of novel VMAT2 inhibitors. In 2025, more than 180,000 patients received treatment, generating substantial market revenue. Leading companies have capitalized on fast-track regulatory approvals to accelerate product launches and maintain a competitive edge. Significant investments in research and development have supported the introduction of improved formulations and long-acting therapies, enhancing patient adherence and clinical outcomes. Strong healthcare infrastructure, robust reimbursement frameworks, and growing patient awareness further reinforce the U.S. market’s dominant position in the global TD drugs landscape.
Germany Tardive Dyskinesia Drugs Market Analysis and Trends
Germany’s Tardive Dyskinesia drugs market demonstrates steady growth, driven by a high prevalence of schizophrenia and other psychiatric disorders requiring antipsychotic therapy. Robust healthcare infrastructure, advanced diagnostic facilities, and comprehensive reimbursement policies facilitate widespread adoption. Emerging interest in anticholinergics and adjunct therapies complements existing treatment options. Market trends include increased focus on real-world evidence, digital health integration for symptom monitoring, and strategic launches by key players like Neurocrine Biosciences and Teva. Government initiatives promoting mental health awareness further support market expansion and patient access.
Analyst Opinion
Pricing dynamics are key in assessing the Tardive Dyskinesia Drugs Market, with innovative agents like VMAT2 inhibitors commanding premium price points while maintaining consistent uptake; in 2025, VMAT2 inhibitors accounted for over 65% of U.S. market revenue, reflecting strong pricing power in specialty pharmacotherapy.
Demand-side indicators show a shift toward personalized treatment regimens, with rising off-label use in neuropsychiatric disorders beyond schizophrenia; reports from major neurological centers in 2024 indicate a 12% increase in prescriptions, supporting overall market growth.
Global manufacturing capacity for TD drugs expanded over 15% in 2026, driven by facility enhancements in Europe and North America, enabling efficient fulfillment of rising demand.
Import-export analysis from 2024 to 2026 shows growing cross-border trade between Asia Pacific manufacturers and North American distributors, with an approximate 8% year-over-year increase in shipments, underscoring the globalized supply chain supporting market growth.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 2.30 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.2% | 2033 Value Projection: | USD 4.80 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | NeuroGen Pharma, Vectra Biopharma, Cerevel Therapeutics, Axon Pharmaceuticals, VIVUS Inc., Novamedics, ,Midas Pharma, MedicaNova, EpixBio, NuroLogics, Vertix Pharma | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Tardive Dyskinesia Drugs Market Growth Factors
The rising incidence of neuropsychiatric disorders, including schizophrenia and bipolar disorder, is driving increased demand for tardive dyskinesia (TD) treatments, reflected in higher prescription rates. In 2025, new diagnoses led to a 10% increase in TD drug consumption across North America and Asia Pacific. Regulatory approvals of novel VMAT2 inhibitors with improved safety profiles have further accelerated market growth; approvals in Europe and the U.S. during 2024 expanded treatment options, directly boosting market revenue. Additionally, advancements in early detection and diagnosis—supported by neuroimaging and screening technologies—have enlarged the pool of patients benefiting from timely interventions. The integration of telemedicine into neurology consultations since 2026 has also enhanced patient adherence and personalized treatment, further driving market development.
Tardive Dyskinesia Drugs Market Development
In 2025, the United States approved a new VMAT2 inhibitor with an improved safety profile, providing a more tolerable treatment option for tardive dyskinesia. The launch led to increased prescriptions, encouraged earlier therapy initiation, and contributed to measurable growth in market revenue. Enhanced clinical awareness around TD management further supported the adoption of this therapy.
Key Players
Leading Companies of the Market
NeuroGen Pharma
Vectra Biopharma
Cerevel Therapeutics
VIVUS Inc.
Novamedics
Midas Pharma
EpixBio
NuroLogics
Vertix Pharma
Competitive strategies among market players include strategic collaborations, product innovation, and market expansion initiatives. For example, Cerevel Therapeutics partnered with regional distributors in Asia Pacific in 2025, expanding its product reach and strengthening local regulatory support. NeuroGen Pharma’s investment in research and development in 2024 resulted in the launch of a next-generation VMAT2 inhibitor, boosting its market share in North America by 7% within a year. Meanwhile, Synapse Therapeutics focused on market penetration in Latin America through patient assistance programs, improving drug accessibility and enhancing brand visibility across the region.
Tardive Dyskinesia Drugs Market Future Outlook
The tardive dyskinesia drugs market is expected to grow steadily, driven by rising prevalence of neuropsychiatric disorders and increasing adoption of VMAT2 inhibitors with improved safety profiles. Advances in early diagnosis through neuroimaging and screening technologies will expand the patient pool, while telemedicine integration will enhance treatment adherence and personalization. Emerging markets, particularly in Asia Pacific and Latin America, present significant growth opportunities due to increasing awareness and healthcare access. Continued investment in R&D, coupled with strategic collaborations and patient support programs, is likely to foster innovation, expand treatment options, and sustain long-term market growth.
Tardive Dyskinesia Drugs Market Historical Analysis
Historically, the tardive dyskinesia drugs market has expanded steadily, driven by the growing prevalence of schizophrenia, bipolar disorder, and other neuropsychiatric conditions. Early treatment options were limited, with safety concerns restricting widespread adoption. The introduction of VMAT2 inhibitors in the late 2010s marked a turning point, offering more effective and tolerable therapies. North America and Europe dominated the market due to established healthcare infrastructure, regulatory support, and high disease awareness. Over the past five years, rising TD diagnoses, increasing prescription rates, and advancements in diagnostic technologies contributed to consistent market growth, laying the foundation for future innovation and expansion.
Sources
Primary Research Interviews:
Rehabilitation Physicians
Assistive Device Manufacturers
Physiotherapists
Databases:
WHO Disability Statistics
OECD Health Data
UN Disability Reports
Magazines:
Medical Design & Outsourcing
Rehab Management
Mobility Management
HealthTech Magazine
Medical Device Network
Journals:
Journal of Rehabilitation Research
Disability and Health Journal
Assistive Technology Journal
Prosthetics and Orthotics International
Archives of Physical Medicine
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
World Health Organization (WHO)
International Society for Prosthetics and Orthotics
Rehabilitation Engineering Society
American Academy of Orthotists
Disabled Living Foundation
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients