U.S. Meibomian Gland Dysfunction Market is estimated to be valued at USD 4,059.8 Mn in 2025 and is expected to reach USD 11,199.8 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 15.6% from 2025 to 2032.
Analysts’ Views on U.S. Meibomian Gland Dysfunction Market:
The increasing research and development (R&D) activities for meibomian gland dysfunction, and increasing prevalence of meibomian gland dysfunction which is expected to propel the market growth over forecast period .
Figure 1. U.S. Meibomian Gland Dysfunction Market Share (%), By Drug Type, 2025
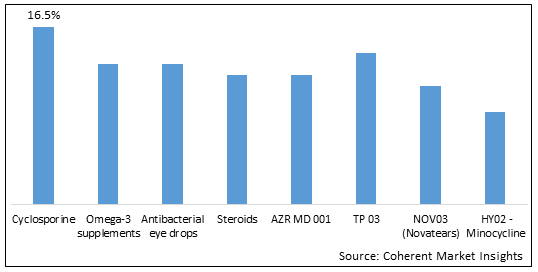
To learn more about this report, Download Free Sample
U.S. Meibomian Gland Dysfunction Market – Driver
Figure 2. . U.S. Meibomian Gland Dysfunction Market Share (%), By Distribution Channel, 2025
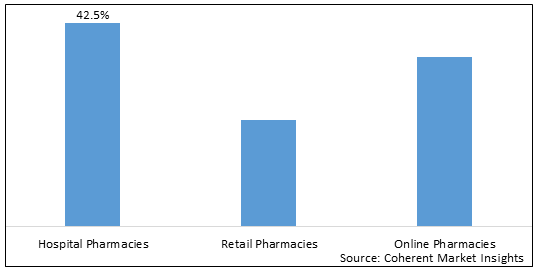
To learn more about this report, Download Free Sample
U.S. Meibomian Gland Dysfunction Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the U.S. Meibomian Gland Dysfunction market. During the lockdown, people's screen time (irrespective of their age) increased significantly, which had a severe impact, and an increase in ophthalmic disorders was observed. For instance, a research study published in December 2021 stated that both dry eye disease (DED) patients and healthy participants up to the age of 60 experienced worsening dry-eye symptoms during the COVID-19 lockout due to increased visual display terminal (VDT) period. The study also reported that younger persons had more severe dry-eye problems than older respondents, particularly during the lockdown. Therefore, the market gained traction as the restrictions were lifted. Since the pandemic led to an increase in dry eye disease cases globally. Hence, it is expected to propel the market growth during the forecast period.
U.S. Meibomian Gland Dysfunction Market Segmentation:
The U.S. meibomian gland dysfunction market report is segmented into Drug Type, By Route of Administration, and By Distribution Channel
U.S. Meibomian Gland Dysfunction Market: Key Developments
U.S. Meibomian Gland Dysfunction Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 4,059.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15.6% | 2032 Value Projection: | USD 11,199.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Major players operating in the U.S. Meibomian Gland Dysfunction Market include Pfizer, Inc., AbbVie Inc., Johnson and Johnson Vision Care Inc., Bausch Health Companies Inc., Novartis AG, Santen Pharmaceutical Co., Ltd., I-MED Pharma Inc., OASIS Medical, Sentiss Pharma Pvt. Ltd., RegeneRx, Akorn, Inc. AFT Pharmaceuticals, Alcon Inc., Horus Pharma, Mitotech, Novaliq GmbH, Otsuka Pharmaceutical Co. Ltd, Prestige Consumer Healthcare, Santen Pharmaceutical Co. Ltd, , Sun Pharmaceutical Industries Ltd, and VISUfarma. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Meibomian Gland Dysfunction Market: Key Trends
People of various ages found living in this multi-screen world
People of various ages are found living in this multi-screen world. Computer or digital screen use may cause less blinking, which may contribute to symptoms of dry eye disease (DED), thereby, increasing the demand for better and more effective therapeutics against DED which is expected to fuel growth in the studied market in the North America region. For instance, an article published in Cureus Journal in July 2022 stated that the prevalence of DED has been estimated at 21% in Canada. Women who used eye cosmetics were substantially more likely to have DED than the general population, which suggests that using eye cosmetics is one of the risk factors for developing DED. The high prevalence of the disease is estimated to boost market growth in the region during the forecast period.
U.S. Meibomian Gland Dysfunction Market: Restraint
Potential side effects of drugs
Potential side effects of drugs, such as steroids, used in the treatment of meibomian gland dysfunction syndrome have a wide range of side effects on the body of the patient who is continuously taking more than the prescribed dose of steroids for a longer period. This is expected to restrain the market growth during the forecast period. For Instance, according to an article published in the National Library of Medicine on April 05, 2023, Reported T-cell activation, immune checkpoint inhibitors are associated with multiple immune-related adverse events, one of which is immune checkpoint inhibitor-induced sicca syndrome. Exocrine gland damage leading to dry mouth and dry eyes were reported as adverse events following the use of nivolumab, durvalumab, and pembroliuzmab. Rheumatic complications were well reported in the literature, especially with regard to the sicca symptoms.
Market Players are taking initiatives for the safety and efficacy of drugs.
U.S. Meibomian Gland Dysfunction Market - Key Players
Major players operating in the U.S. Meibomian Gland Dysfunction market include Pfizer, Inc., AbbVie Inc., Johnson and Johnson Vision Care Inc., Bausch Health Companies Inc., Novartis AG, Santen Pharmaceutical Co., Ltd., I-MED Pharma Inc., OASIS Medical, Sentiss Pharma Pvt. Ltd., RegeneRx, Akorn, Inc. AFT Pharmaceuticals, Alcon Inc., Horus Pharma, Mitotech, Novaliq GmbH, Otsuka Pharmaceutical Co. Ltd, Prestige Consumer Healthcare, Santen Pharmaceutical Co. Ltd, Sun Pharmaceutical Industries Ltd, and VISUfarma.
*Definition: Meibomian gland dysfunction is a cause of dry eye syndrome (DES), also known as keratisis sicca and keratoconjunctivitis sicca. Patients with DES suffer damage to the ocular surface, instability in the tear film, and visual disturbance. Tear film covers the ocular surface, which is made up of three intertwined layers, a superficial lipid layer, produced by meibomian glands, which assists in reducing tear evaporation and uniform tear spreading, middle thick aqueous layer produced from lacrimal glands, and the innermost hydrophilic mucin layer produced from goblet cells of conjunctiva and epithelium of ocular surface.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients