The Gonadotropin-releasing hormone (GnRH) agonists are considered to have greater safety and efficacy profile than anti-androgens and estrogens and are among various therapies of achieving medical castration. Furthermore, the drug is mostly implanted in the body in the form of slow-release formulations which make the patient comfortable, improve the quality of life, and also reduce cost of the treatment. Most of the time, prostate cancer progresses with increasing testosterone hormone. Moreover, the production of testosterone can be stopped by surgically removing the testicles or through medication therapy. Triptorelin is GnRH agonist which is designed to stop the tentacles from making testosterone. This eventually reduces testosterone levels and minimizes the progression of prostate cancer.
The U.S. triptorelin market was valued at US$ 171.7 Mn in 2022 and is expected to exhibit a CAGR of 5.5% over the forecast period (2022-2030).
U.S. Triptorelin Market - Impact of the Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic is expected to drive the growth of the U.S. triptorelin market over the forecast period, as market players are engaged in making the triptorelin available for the patients during the COVID-19 pandemic. For instance, in the year 2020, Arbor Pharmaceuticals, LLC, a U.S.-based specialty pharmaceutical company announced the full availability of Triptodur (triptorelin) in the U.S., two times a year during the COVID-19 pandemic. Triptodur (triptorelin) is an injectable gonadotropin releasing hormone agonist (GnRHa), which is indicated for the treatment of pediatric patients two years of age and older and are suffering from central precocious puberty (CPP).
Moreover, in the year 2020, Arbor Pharmaceuticals, LLC, confirmed that it has ordered supply of Triptodur (triptorelin) for its partner Debiopharm, in order to avoid the shortage during the COVID-19 pandemic.
Figure 1: U.S. Triptorelin Market Share (%) Analysis, By Product Type, 2022
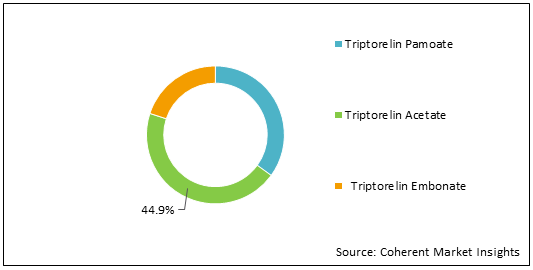
To learn more about this report, Download Free Sample
Manufacturers are providing reimbursement for the drug triptorelin and other resources, this is expected to attract the patients for choosing triptorelin for their treatment. This will drive the growth of the U.S. triptorelin market over the forecast period.
For instance, in the year 2022, Arbor Pharmaceuticals, LLC, subsidiary of Azurity Pharmaceuticals, Inc. is currently offering reimbursement for the patients undergoing treatment for central precocious puberty (CPP) with Triptodur (triptorelin). The company is providing the drug at a low cost so that all the patients can afford the drug. Under this reimbursement policy, the patient can save almost US$ 10,000 per year. The offer is valid in the U.S. and Puerto Rico as allowed by law. Under the policy, only those patients are eligible for the reimbursement policy, who are insured in whole or in part, by Medicaid, Medicare, or other federal or state healthcare programs.
U.S. Triptorelin Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 171.7 Mn |
| Historical Data for: | 2017 to 2021 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 5.5% | 2030 Value Projection: | US$ 274.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Ipsen USA, Bachem Americas, Inc., Ferring Pharmaceuticals USA, Dr. Reddy’s Laboratories Ltd, Teva Pharmaceutical Industries Ltd., Arbor Pharmaceuticals, LLC, Watson Laboratories, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Many government organizations are conducting research and development activities, in order to study triptorelin for extended application of in various treatment modalities and this is expected to drive the growth of the U.S. triptorelin market during the forecast period.
The drug triptorelin is being tested for various applications, this may increase the growth of the market over the forecast period. For instance, Triptorelin is being studied to analyze effects of adjuvant hormonal therapy treatments of Letrozole + Zoledronate, Letrozole and Tamoxifen, and on bone loss in breast cancer patients. According to the clinical trials website, in July 2017, the study was sponsored by National Cancer Institute (NCI), Naples; and are present in phase III clinical stage. Moreover, according the same source, triptorelin market shows lucrative revenue growth in the near future, with the successful completion of this study and potential launch of new products during the forecast period.
U.S. Triptorelin Market – Restraints
U.S. triptorelin market growth is expected to be hampered in the near future, owing to side effects that have been recorded by the U.S. Food and Drug Administration (FDA). For instance, the U.S. FDA, in the year 2019, updated the side-effects for triptorelin that have been observed in the patients who are taking these medicines. They worsen the symptoms during tumor flare, hot flushes, decreased libido and impotence, bone and joint pain (may be increased initially), injection site reactions (pain, mild bruising, and erythema), sweating and weight gain.
Figure 2: U.S. Triptorelin Market Value (US$ Mn) & Y-o-Y Growth (%), 2022 – 2030
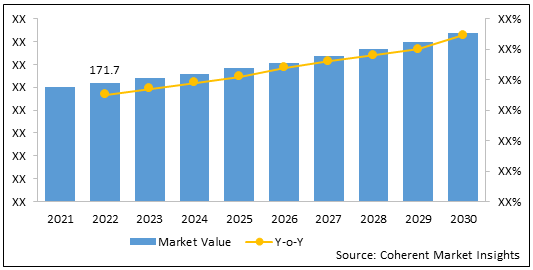
To learn more about this report, Download Free Sample
U.S. Triptorelin Market – Competitive Landscape
Major players operating in the U.S. triptorelin market include Aero Medical Ambulance Service, Aero-Dienst GmbH, Airlec Air Espace, U.S.an Air Ambulance, Flightserve International, IAS Medical, Medical Air Service, Quick Air Jet Charter GmbH, Babcock Scandinavian Air Ambulance, and Capital Air Ambulance
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients