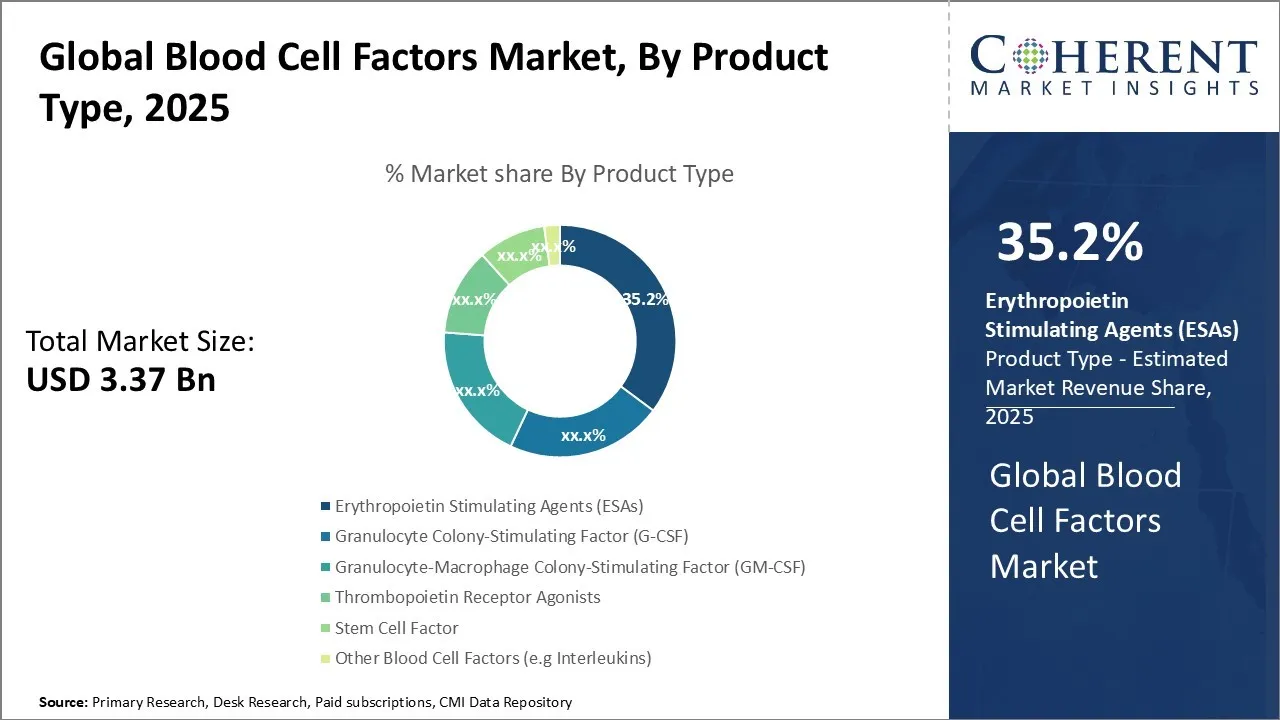
Global Blood Cell Factors Market Size and Forecast – 2025 to 2032
The Global Blood Cell Factors Market is estimated to be valued at USD 3.37 Bn in 2025 and is expected to reach USD 4.31 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 3.6% from 2025 to 2032.
Key Takeaways of the Global Blood Cell Factors Market:
- In 2025, Erythropoietin Stimulating Agents (ESAs) are expected to hold the largest share of 35.2% of the blood cell factors market by product type.
- By source, recombinant (rDNA technology-derived) blood cell factors are projected to lead with a 62.2% share in 2025.
- In terms of application, oncology is expected to dominate the market with a 34.2% share in 2025.
- North America is expected to lead the market, holding a share of 40.3% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with a market share of 32.2% in 2025.
Market Overview:
The global blood cell factors market growth is driven by the increasing prevalence of blood disorders, advancements in healthcare infrastructure, and rising demand for effective treatments. Additionally, growing awareness about blood cell factors and their role in treating various blood-related conditions are expected to fuel the market growth. However, the high cost of treatments and stringent regulatory guidelines may pose challenges to the market's expansion.
Currents Events and Their Impact
|
Current Events |
Description and its impact |
|
Technological Advancements in Blood Cell Factor Production |
|
|
Biotech Investments in Blood Cancer Therapies |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Blood Cell Factors Market Insights, By Product Type – Erythropoietin Stimulating Agents (ESAs) Dominate Due to High Prevalence of Anemia in CKD and Cancer Patients
In terms of Product Type, Erythropoietin Stimulating Agents (ESAs) segment is expected to contribute the highest market share of 35.2% in 2025. ESAs are widely used in the treatment of anemia associated with chronic kidney disease (CKD), cancer, and other conditions characterized by decreased red blood cell production. The high prevalence of CKD and cancer worldwide is a significant driver for the segment growth. According to the National Kidney Foundation, Inc., ESAs have been in use for several decades, and their efficacy and safety profiles are well-established. This long-standing presence in the market has led to a high level of physician familiarity and confidence in prescribing ESAs. Additionally, the development of novel ESAs with improved pharmacokinetic and pharmacodynamic properties, such as longer half-lives and reduced dosing frequencies, has further boosted the adoption of these agents.
Blood Cell Factors Market Insights, By Source – Recombinant Technology Leads Owing to Safety, Consistency, and Reduced Pathogen Risk
In terms of Source, Recombinant (rDNA technology-derived) blood cell factors segment is expected to contribute the highest market share of 62.2% in 2025. The widespread adoption of recombinant technology in the production of blood cell factors has revolutionized the industry, offering several advantages over plasma-derived products. Recombinant factors are manufactured using genetically engineered cell lines, ensuring a consistent and reliable supply of high-quality proteins. This technology eliminates the risk of transmission of blood-borne pathogens associated with plasma-derived products, enhancing patient safety.
Blood Cell Factors Market Insights, By Application – Oncology Segment Tops the Market Driven by Rising Cancer Burden and Supportive Care Needs
In terms of Application, Oncology segment is estimated to hold the highest market share of 34.2% in 2025. Cancer patients often experience anemia and other hematological complications as a result of the disease itself or the side effects of chemotherapy and radiation therapy. Blood cell factors, particularly ESAs and G-CSF, play a crucial role in managing these complications, improving patient outcomes and quality of life.
The increasing global burden of cancer is a significant driver for the growth of the oncology segment. According to the World Health Organization, cancer is the second leading cause of death worldwide, with an estimated 19.3 million new cases and 10 million cancer-related deaths in 2020. As cancer incidence rates continue to rise, the demand for effective supportive care, including the use of blood cell factors, is expected to grow in parallel.
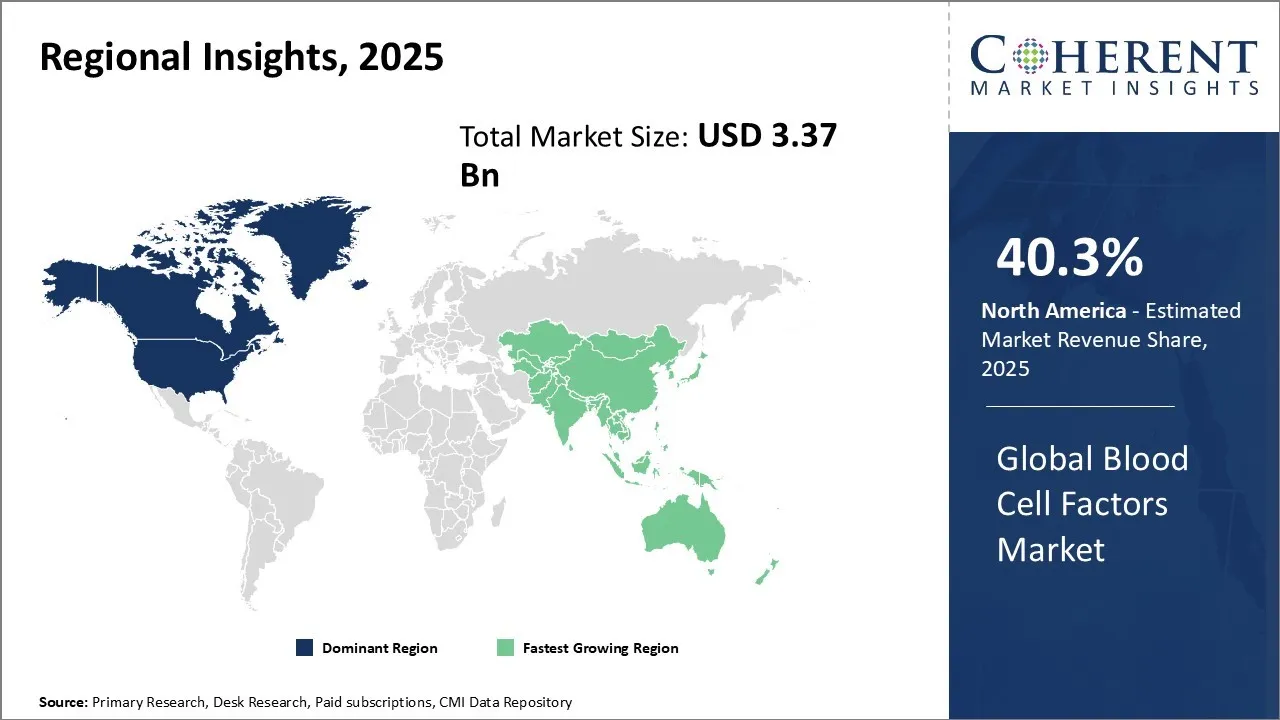
Regional Insights
To learn more about this report, Download Free Sample
North America Blood Cell Factors Market Analysis and Trends
North America dominates the global blood cell factors market with an estimated share of 40.3% in 2025. The region boasts a well-established healthcare infrastructure, advanced research facilities, and a strong presence of key market players. The U.S., in particular, has been at the forefront of medical innovation, with numerous companies dedicating significant resources to the development of blood cell factors. The favorable regulatory environment and supportive government policies have further encouraged investment in research and development, driving the market growth. Additionally, the high prevalence of blood disorders and the increasing adoption of regenerative medicine treatments have contributed to the dominance of North America in this market For instance, Terumo Blood and Cell Technologies (Terumo BCT), a global leader in blood management and cellular therapy solutions, introduced its Reveos Automated Blood Processing System in the U.S. The system automates the separation of whole blood into platelets, red cells, and plasma, boosting efficiency and consistency over manual methods.
Asia Pacific Blood Cell Factors Market Analysis and Trends
The Asia Pacific region exhibits the fastest growth in the global blood cell factors market with 32.2% in 2025. This growth can be attributed to the rapidly expanding healthcare sector, increasing government initiatives to improve healthcare access, and rising awareness about advanced medical treatments. Countries like China and India have witnessed significant economic growth, leading to increased healthcare spending and a growing middle-class population. This, in turn, has fueled the demand for blood cell factors and related treatments. Moreover, the presence of a large patient pool, coupled with the improving healthcare infrastructure and increasing collaboration between international and local companies, has further accelerated the market growth in the Asia Pacific region.
Global Blood Cell Factors Market Outlook for Key Countries
U.S. Blood Cell Factors Market Trends
The U.S. blood cell factors market is driven by a combination of factors, including a robust healthcare system, advanced research capabilities, and a strong presence of key market players. Companies such as Amgen, Johnson & Johnson, and Gilead Sciences have made significant contributions to the development and commercialization of blood cell factors in the country.
For instance, in January 2025, Exicure, a U.S.-based biotech recently revived under new management, acquired the U.S. subsidiary of South Korea’s GPCR Therapeutics in a strategic move to re-enter the clinical space. The deal includes burixafor, a CXCR4 antagonist in Phase 2 trials for blood cancer, being tested alongside propranolol and G-CSF to boost white blood cell production. Early trial data has shown promise, with potential for a Phase 3 trial ahead.
China Blood Cell Factors Market Trends
China's blood cell factors market is experiencing rapid growth, driven by increasing government support, rising healthcare expenditure, and a large patient population. The country has made significant strides in improving its healthcare infrastructure and promoting research and development in the field of blood cell factors. Local companies, such as Sihuan Pharmaceutical and 3SBio, have emerged as key players in the market, while international collaborations have facilitated technology transfer and market expansion.
For instance, in December 2024, Takeda, a global biopharmaceutical leader, signed an exclusive licensing deal with Keros Therapeutics, a clinical-stage biotech firm, to develop and commercialize elritercept outside mainland China, Hong Kong, and Macau. Elritercept is a promising investigational activin inhibitor targeting activin A and B, key proteins linked to anemia-related diseases.
Germany Blood Cell Factors Market Trends
Germany's blood cell factors market is experiencing robust growth, driven by advancements in hematology diagnostics, biopharmaceutical innovations, and a strong emphasis on preventive healthcare. In March 2025, according to new research by The German Cancer Research Center (DKFZ)—Germany’s largest biomedical research institution—frequent blood donations lead to genetic adaptations in blood stem cells that enhance their regenerative capacity. The study, conducted in collaboration with HI-STEM and the German Red Cross Blood Donor Service, highlights that while aging often results in "clonal hematopoiesis"—an accumulation of mutated stem cell clones linked to diseases—regular blood donations may activate protective mechanisms that support healthier blood cell renewal over time.
Japan Blood Cell Factors Market Trends
Japan's market for blood cell factors is characterized by a high level of technological advancement, a rapidly aging population, and increasing healthcare expenditure. The country's leading pharmaceutical companies, such as Takeda and Astellas, have been actively involved in the development and commercialization of blood cell factors. Japan's stringent regulatory environment and focus on quality have ensured the delivery of safe and effective treatments to patients.
End User Feedback and Unmet Needs – Global Blood Cell Factors Market
- End users across hospitals, specialty clinics, and research institutions have reported growing satisfaction with next-generation blood cell factors, particularly recombinant erythropoietin and G-CSF therapies. Positive feedback centers on improved efficacy, reduced administration frequency, and better patient tolerance. For instance, The German Cancer Research Center highlighted the integration of long-acting biosimilars in chemotherapy-induced neutropenia protocols, noting a 20% drop-in hospitalization rates due to fewer febrile neutropenia episodes. Such outcomes reinforce confidence in therapeutic performance and support broader clinical adoption.
- Conversely, recurring challenges persist. End-users in low-resource settings, especially in parts of Asia and Africa, report affordability and cold-chain maintenance as major barriers. A government-run hospital in India cited inconsistent access to thrombopoietin analogs due to procurement delays and cost constraints, which impacted timely treatment of aplastic anemia patients. Across the board, unmet needs include demand for temperature-stable formulations, flexible dosing schedules, and region-specific clinical guidelines. Addressing these gaps could not only improve health equity but also open new market opportunities through localized manufacturing, enhanced training programs, and strategic public-private collaborations. Manufacturers who respond to these concerns are likely to see increased customer retention and long-term market growth.
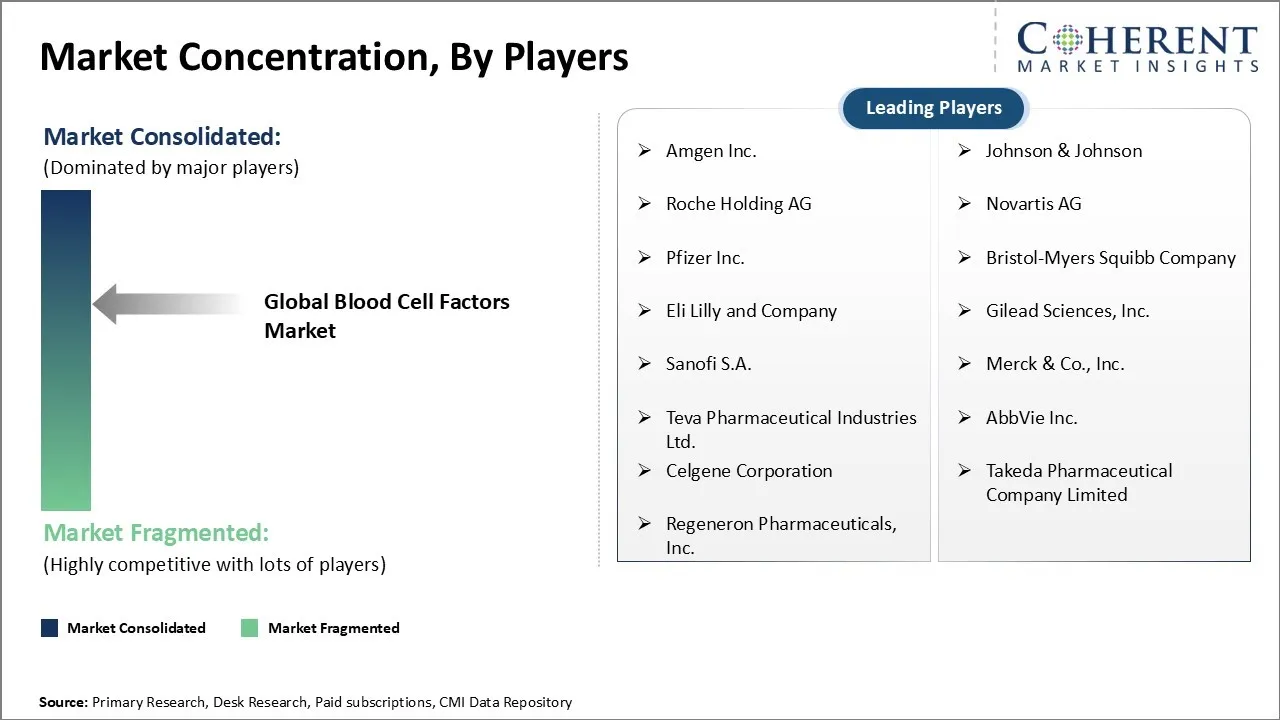
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments:
- In February 2025, Novartis, a global healthcare company based in Switzerland known for pioneering innovative medicines, announced the USD 925 million acquisition of Anthos Therapeutics, a U.S-based startup it co-founded with Blackstone Life Sciences. The deal could reach USD 3.1 billion with milestone payments. Through this acquisition, Novartis regains full rights to abelacimab, a Factor XI inhibitor currently in Phase 3 trials, aimed at offering a next-generation blood-thinning therapy with potentially fewer bleeding risks.
- In April 2023, the U.S. FDA approved Omisirge (omidubicel-onlv), a cord blood-based cell therapy developed by Gamida Cell, a biotech company specializing in advanced cellular therapies for blood cancers and serious hematologic conditions. Approved for patients aged 12 and above undergoing umbilical cord blood transplantation after intensive treatment, Omisirge accelerates neutrophil recovery, helping reduce the risk of severe infections following stem cell transplants in blood cancer patients.
- In February 2023, Agios Pharmaceuticals, Inc., a biotech company focused on therapies for rare diseases driven by cellular metabolism, announced the formation of a multi-stakeholder advocacy advisory council for hemolytic anemias, including PK deficiency, thalassemia, and sickle cell disease. The council—comprising patients, caregivers, and physicians—aims to address shared challenges such as disease burden, care transition issues, and quality of life, while also generating evidence-based insights and raising awareness across these conditions.
Top Strategies Followed by Global Blood Cell Factors Market Players
- Established Players in the global blood cell factors market are focusing on extensive research and development to innovate high-performance products. These companies are investing heavily in R&D to develop advanced blood cell factors that offer superior efficacy and safety profiles. By dedicating resources to scientific research, these players aim to stay ahead of the competition and maintain their market leadership.
- For example, companies such as Amgen Inc., Johnson & Johnson, and Sanofi, are heavily investing in R&D to innovate advanced biologics like long-acting erythropoietin and granulocyte colony-stimulating factors (G-CSF). These companies aim to enhance efficacy, reduce dosage frequency, and improve patient adherence.
- Mid-Level Players in the global blood cell factors market are adopting strategies focused on delivering quality, budget-friendly products targeting price-sensitive consumers. These companies recognize the importance of offering cost-effective solutions to capture a larger market share. By optimizing their manufacturing processes and sourcing strategies, mid-level players can provide competitive pricing without compromising on product quality. Furthermore, these players are exploring collaborations with other companies to boost their technology, production capabilities, and market presence.
- For example, in April 2020, Biocon Ltd. and Mylan N.V.,a global pharmaceutical company, announced the launch of Fulphila, a biosimilar to Neulasta (pegfilgrastim), in Canada. Approved by Health Canada, Fulphila is indicated to reduce the risk of infection, such as febrile neutropenia, in patients with non-myeloid cancers undergoing myelosuppressive chemotherapy.
- Small-Scale Players in the global blood cell factors market are targeting niche markets with unique features or innovative products. These companies focus on specialization to differentiate themselves from larger competitors. By identifying specific market needs and developing tailored solutions, small-scale players can carve out a profitable niche.
- For example, Orca Bio is pioneering precision cell therapies for blood disorders, targeting specific immune responses—a niche segment underserved by larger biologics. On a more regional level, Emcure Biotech in India focuses on hematology support products in emerging markets, aided by alliances with local distributors and manufacturers.
Market Report Scope
Blood Cell Factors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.37 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 3.6% | 2032 Value Projection: | USD 4.31 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., Johnson & Johnson, Roche Holding AG, Novartis AG, Pfizer Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, Gilead Sciences, Inc., Sanofi S.A., Merck & Co., Inc., Teva Pharmaceutical Industries Ltd., AbbVie Inc., Celgene Corporation, Takeda Pharmaceutical Company Limited, and Regeneron Pharmaceuticals, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
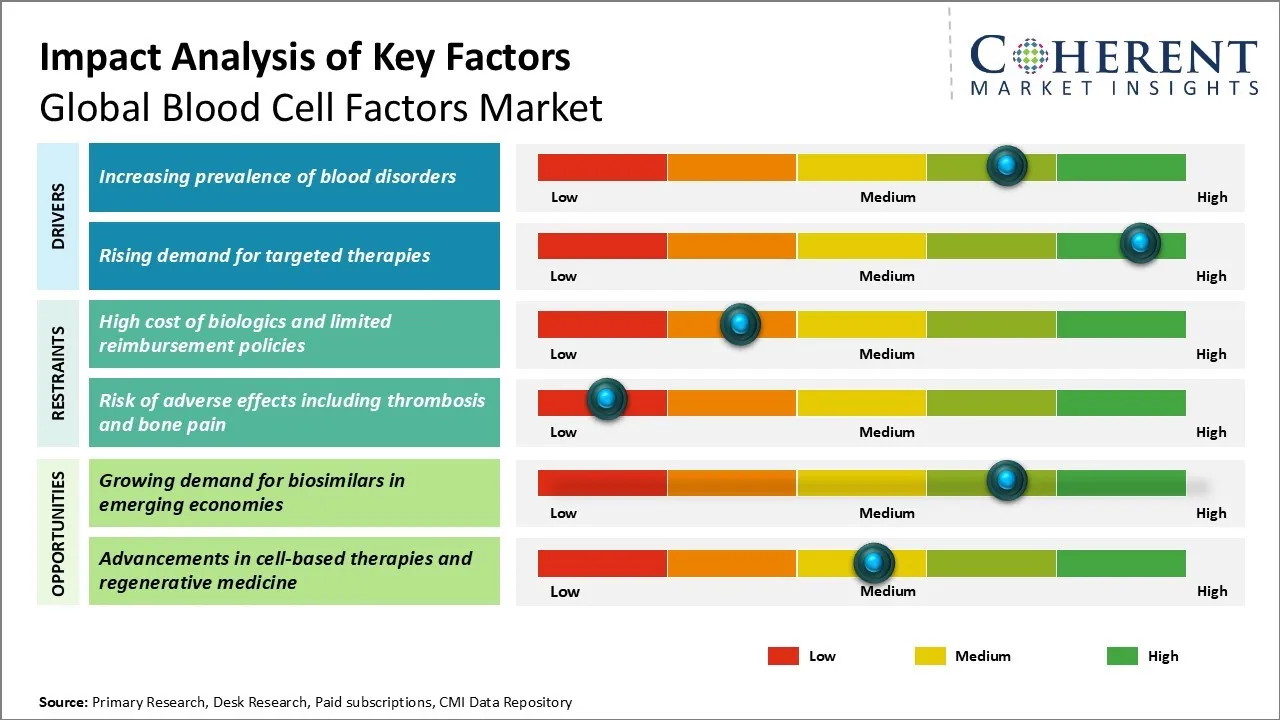
Blood Cell Factors Market Dynamics
To learn more about this report, Download Free Sample
Blood Cell Factors Market Driver - Increasing prevalence of blood disorders
The global blood cell factors market is significantly driven by the increasing prevalence of various blood disorders worldwide. Conditions such as anemia, leukemia, lymphoma, and hemophilia are becoming more common, leading to a higher demand for blood cell factors. A key driver of the global blood cell factors market is the advancement of gene therapies for blood disorders. These therapies offer long-term or potentially curative outcomes by enabling the sustained production of essential blood cell factors, reducing the need for frequent infusions. Such innovations are reshaping treatment approaches, improving patient outcomes, and significantly increasing the demand for advanced blood cell factor products worldwide
For instance, in December 2024, according to release by the Ministry of Health and Family Welfare, India’s first in-human gene therapy for Severe Hemophilia A using a lentiviral vector has shown promising results—achieving zero annual bleeding rate in all five trial participants and enabling sustained production of Factor VIII, eliminating the need for repeated infusions. This milestone therapy was developed by the Centre for Stem Cell Research (CSCR) at CMC Vellore, a translational unit under BRIC-inStem, with support from the Department of Biotechnology.
Blood Cell Factors Market Opportunity: Growing demand for biosimilars in emerging economies
The growing demand for biosimilars in emerging economies presents a significant opportunity for the Global Blood Cell Factors Market. Biosimilars are biologic products that are highly similar to approved reference biologics, offering comparable safety and efficacy at a lower cost. As emerging economies face the burden of increasing healthcare costs and the need to expand patient access to essential treatments, the demand for affordable alternatives like biosimilars is on the rise. These markets, with their large patient populations and evolving regulatory frameworks, provide a fertile ground for the entry and expansion of biosimilar blood cell factors.
Moreover, the development of local manufacturing capabilities and partnerships with regional stakeholders can further drive the adoption of biosimilars in emerging economies. For Example, India, with one of the most active biosimilar markets globally, has seen companies like Biocon Biologics, Dr. Reddy’s Laboratories, and Intas Pharmaceuticals introduce cost-effective biosimilar versions of epoetin alfa and filgrastim, widely used in treating anemia and chemotherapy-induced neutropenia. Dr. Reddy’s biosimilar of pegfilgrastim (a long-acting G-CSF), marketed under the brand name Pelgraz, is not only approved in India but also in several EU markets, demonstrating how emerging-market manufacturers are gaining global reach.
Analyst Opinion (Expert Opinion)
- The global blood cell factors market is witnessing steady growth, driven by rising incidences of hematologic disorders, increasing use of recombinant therapies, and advancements in gene and cell therapy technologies. Regulatory support for rare disease treatments, growing investment in biologics manufacturing, and enhanced awareness about early diagnosis have further propelled demand. However, the market continues to face challenges such as high treatment costs, limited accessibility in low-resource regions, and concerns regarding long-term safety of biologics. Opportunities lie in expanding biosimilar adoption, particularly in emerging markets, and leveraging AI-driven diagnostics to optimize therapy outcomes.
- Notable developments include India’s first human gene therapy for Hemophilia A by CSCR-Vellore and the USFDA approval of Omisirge, a cord blood-derived cell therapy for blood cancer patients. Events like the American Society of Hematology (ASH) Annual Meeting, ISCT Global Conference, and World Congress on Blood Disorders have played a vital role in shaping industry discourse and innovation pathways. These platforms facilitate global collaboration, showcase emerging technologies, and support policy frameworks that enhance therapeutic access and scalability across regions.
Market Segmentation
- Product Type Insights (Revenue, USD Bn, 2020 - 2032)
-
- Erythropoietin Stimulating Agents (ESAs)
- Granulocyte Colony-Stimulating Factor (G-CSF)
- Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
- Thrombopoietin Receptor Agonists
- Stem Cell Factor
- Other Blood Cell Factors (e.g. Interleukins)
- Source Insights (Revenue, USD Bn, 2020 - 2032)
-
- Recombinant (rDNA technology-derived)
- Natural (Plasma-derived)
- Application Insights (Revenue, USD Bn, 2020 - 2032)
-
- Oncology
- Nephrology
- Hematology Disorders
- Infectious Diseases
- Autoimmune Disorders
- Others (e.g., HIV-related anemia)
- Route Of Administration Insights (Revenue, USD Bn, 2020 - 2032)
-
- Injectable
- Oral
- End User Insights (Revenue, USD Bn, 2020 - 2032)
-
- Hospitals
- Ambulatory Surgical Centers
- Home Healthcare
- Specialty Clinics
- Research & Academic Institutes
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
-
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
-
- Amgen Inc.
- Johnson & Johnson
- Roche Holding AG
- Novartis AG
- Pfizer Inc.
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- Gilead Sciences, Inc.
- Sanofi S.A.
- Merck & Co., Inc.
- Teva Pharmaceutical Industries Ltd.
- AbbVie Inc.
- Celgene Corporation
- Takeda Pharmaceutical Company Limited
- Regeneron Pharmaceuticals, Inc.
Sources
Primary Research Interviews:
- Hematologists and oncologists in leading hospitals (e.g., Mayo Clinic, AIIMS)
- Product managers from biopharma companies producing blood cell factors
- Clinical researchers and principal investigators from Phase II/III blood factor trials
- Hospital procurement officers and public health administrators
- Regulatory affairs experts and medical officers from national health bodies
Databases:
- World Health Organization (WHO)
- U.S. Food and Drug Administration (FDA)
- European Medicines Agency (EMA)
- gov (NIH)
- Centers for Disease Control and Prevention (CDC)
- UNICEF & Global Health Data Exchange (GHDx)
- Ministries of Health (e.g., India, Brazil, South Africa)
Magazines:
- BioPharma Dive
- Pharmaceutical Technology
- FiercePharma
- GEN (Genetic Engineering & Biotechnology News)
- Drug Discovery & Development
- Nature Biotechnology News
Journals:
- Blood (American Society of Hematology)
- The Lancet Haematology
- British Journal of Haematology
- Journal of Hematology & Oncology
- Nature Reviews Drug Discovery
- Journal of Clinical Oncology
Newspapers:
- The New York Times – Health Section
- The Guardian – Global Health
- Reuters Health
- BBC Health News
- Stat News (Biotech & Pharma)
Associations:
- American Society of Hematology (ASH)
- European Hematology Association (EHA)
- World Federation of Hemophilia (WFH)
- International Society for Cell & Gene Therapy (ISCT)
- International Society for Stem Cell Research (ISSCR)
Public Domain Sources:
- bioRxiv / medRxiv – Preprint articles on emerging blood factor research
- OpenFDA – Access to labeling, adverse events, and drug recalls
- Data.gov (USA) – Government-funded datasets on healthcare, biologics, and clinical trials
- WHO Essential Medicines List
Proprietary Elements:
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients