Global Coated Tablets Market Size and Forecast – 2026-2033
According to Coherent Market Insights, the global coated tablets market is estimated to be valued at USD 1.04 Bn in 2026 and is expected to reach USD 1.96 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 9.5% from 2026 to 2033. This steady growth reflects increasing demand driven by advancements in pharmaceutical technologies and rising preference for coated tablets due to improved patient compliance, enhanced drug stability, and targeted delivery mechanisms, positioning the market for significant expansion over the forecast period.
Key Takeaways of the Global Coated Tablets Market
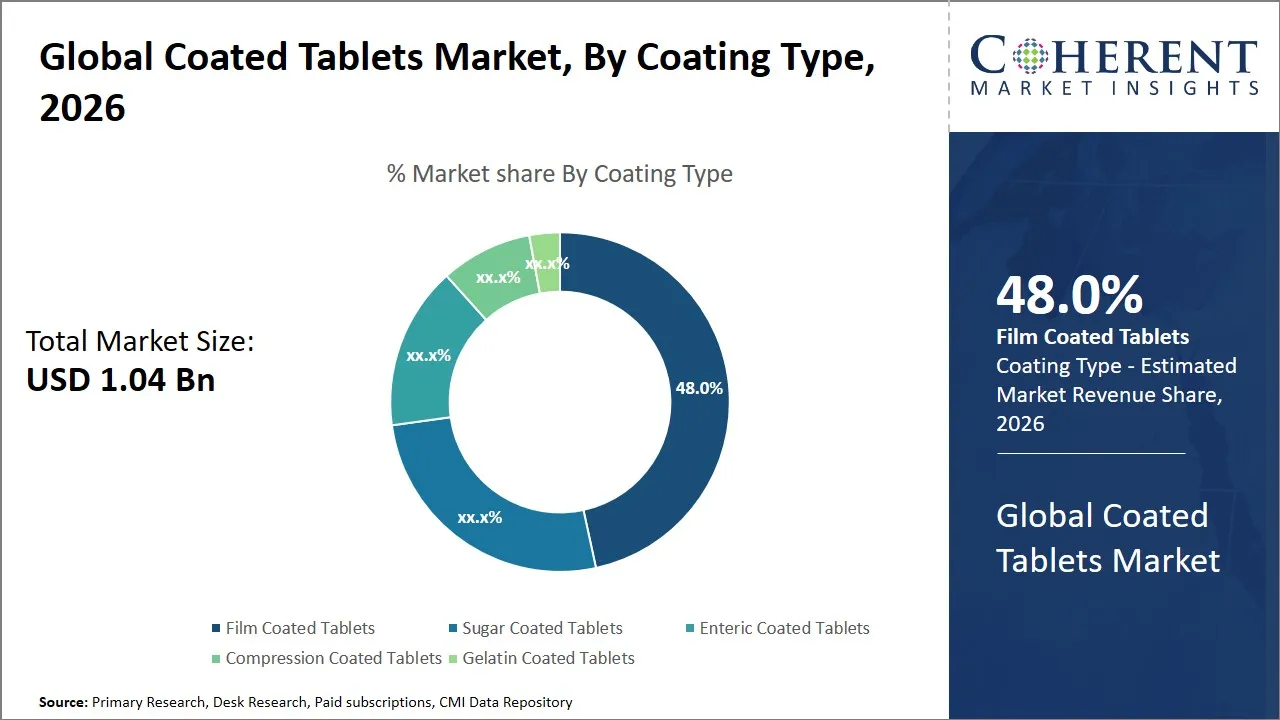
- Film coated tablets segment is expected to lead the global coated tablets market, capturing 48% share in 2026.
- Polymer-based coatings segment is estimated to represent 37% of the coated tablets market share in 2026.
- Immediate release coating segment is projected to dominate with 34% of the global coated tablets market share in 2026.
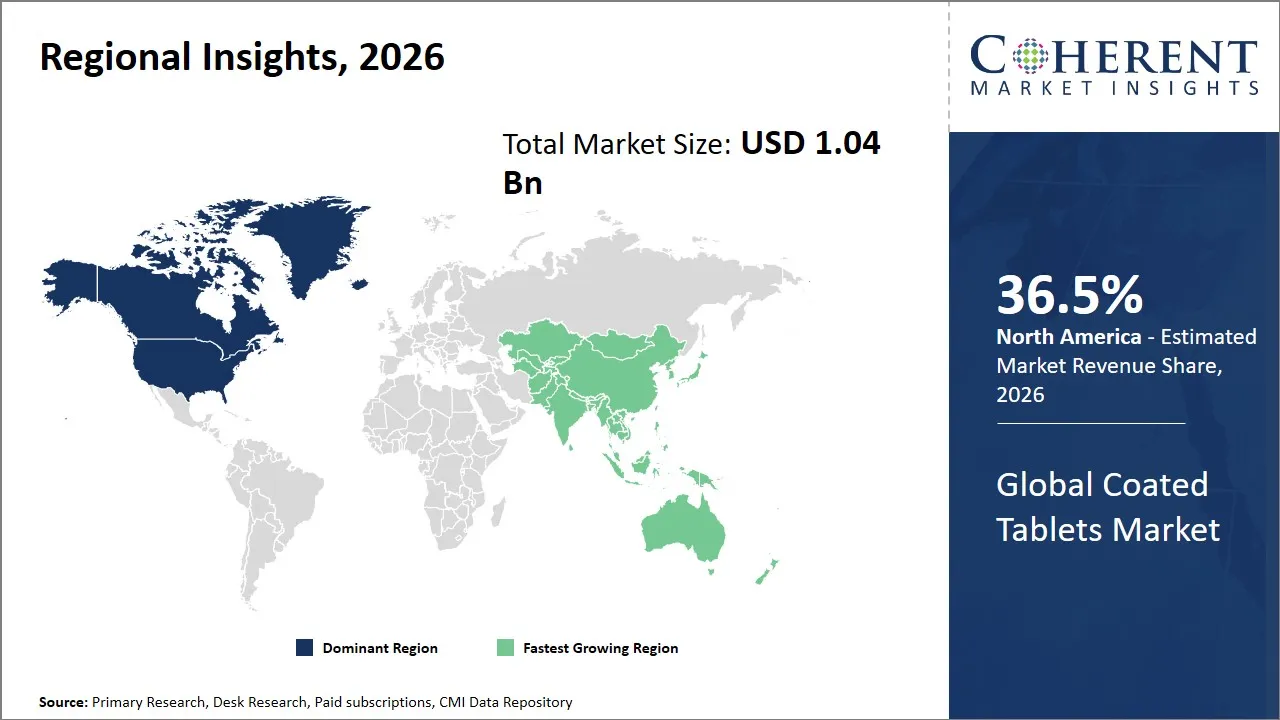
- North America is expected to lead the coated tablets market, holding a share of 36.5% in 2026. Asia Pacific is anticipated to be the fastest-growing region, with 27% share in 2026.
Market Overview
- Rising demand for patient-friendly oral solid dosage forms supporting improved swallowability, taste masking, and drug stability.
- Increasing prevalence of chronic diseases driving long-term consumption of coated oral medications worldwide.
- Expansion of generic drug manufacturing and lifecycle reformulation strategies boosting coated tablet production.
- Growing adoption of modified-release and gastro-resistant coating technologies enhancing therapeutic performance.
Currents Events and their Impact
|
Current Events |
Description and its Impact |
|
Global rise in chronic disease treatment demand |
|
|
Expansion of generic pharmaceutical manufacturing |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Why Does the Film Coated Tablets Segment Dominate the Global Coated Tablets Market in 2026?
The film coated tablets segment is expected to hold the largest share of 48% in 2026, driven by their superior functional properties and wide applicability across therapeutic categories. The popularity of film coating can be explained by the fact that it allows creating a protective barrier, which contributes greatly to the stability of the active pharmaceutical ingredient (API).
This coating prevents the drug to be affected by environmental factors like moisture, oxygen and light which degrades sensitive compounds hence enhancing shelf life and efficacy. Furthermore, the film coating provides enhanced mechanical properties, decreases the friability of the tablet and lowers the damage caused to the tablet during manipulation and transportation.
For instance, in August 2024, Bayer continued to focus on its cardiovascular portfolio, including Aspirin Protect, which is designed to improve gastrointestinal (GI) tolerability during long-term antiplatelet therapy. The product is part of a broader, well-established range of low-dose acetylsalicylic acid formulations used for secondary prevention of cardiovascular events.
(Source- https://www.bayer.com/en/ae/aspirin-protect-in-saudi-arabia#:)
The Polymer-Based Coatings Segment Dominates the Global Coated Tablets Market
The polymer-based coatings segment is projected to capture 37% share in 2026, due to their remarkable versatility and ability to fulfill multiple coating objectives. Polymers have tunable physical and chemical characteristics that enable formulators to tailor coatings to the desired release mechanism, protection or appearance needs. This versatility renders polymer-based coatings to be essential in various pharmaceutical preparations, both immediate and controlled release drugs.
The ability of polymeric coatings to enable the controlled release of drugs is one of the main reasons why the method is considered to be the strength of polymeric coatings. The properties of the polymer, including it molecular weight, hydrophobicity and cross-linking density can be manipulated, allowing manufacturers to develop coatings that dissolve at given pH levels or time intervals, allowing the targeted delivery in the gastrointestinal tract.
For instance, in June 2024, Evonik announced expansion of its pharmaceutical solutions portfolio supporting polymer-based coating technologies, including EUDRAGIT polymers used widely in enteric and controlled-release coated tablets, enabling drug manufacturers to develop targeted release oral solid dosage formulations across global markets.
(Source- https://healthcare.evonik.com/en/news-and-events/press-releases/)
Immediate Release Coating Segment Dominates the Coated Tablets Market
The immediate release coating segment is expected to capture the highest share of 34% in 2026, attributable to their critical role in ensuring rapid and consistent drug bioavailability. And instant-release coats are developed to dissolve rapidly when consumed, such that the active ingredient is soon released and absorbed an essential need in numerous therapeutic applications where speed is required, i.e., pain treatment, acute infections, or symptomatic relief.
Regulatory Approval Delays Limiting Adoption of Advanced Tablet Coating Technologies
- Regulatory approval of new coating technologies can often involve significant amounts of safety, stability and performance data to prove that modified coating do not change drug efficacy, release profile or the safety of patients, thus extending approval timelines around major markets before commercial approval can be achieved.
- Pharmaceutical firms should do more validation, manufacturing procedure qualification and regulatory documentation capabilities on the launch of innovative coating systems, making the process costly in development and delaying the time of product introduction, which lags out the market acceptance of innovative coated technologies of pills.
Regional Insights
To learn more about this report, Download Free Sample
North America Coated Tablets Market Analysis and Trends
The North America region is projected to lead the market with a 36.5% share in 2026, owing to a highly developed pharmaceutical ecosystem, robust healthcare infrastructure, and stringent regulatory frameworks that encourage innovation and high-quality drug manufacturing. The existence of large pharmaceutical firms like Pfizer, Johnson and Johnson, and Merck leads to serious developments of both coated tablet technologies such as controlled-release and taste-masking technologies that enhance patient compliance. The policies by the government that favor drug approvals, patent protection, and expenditure in healthcare enhance the power of the market. Moreover, North America has a well-developed supply chain and solid trading relationships which make distribution of the coated tablets in the region and to the other markets across the globe easily.
For instance, in October 2025, IQVIA reported continued growth in the North America coated tablets market, driven by increased prescriptions of film-coated and controlled-release oral formulations for chronic conditions such as diabetes and cardiovascular diseases, with demand particularly strong in the U.S. and Canada due to aging populations and preference for patient-friendly dosage forms.
Asia Pacific Coated Tablets Market Analysis and Trends
The Asia Pacific region is expected to exhibit the fastest growth in the coated tablets market, contributing 27% share in 2026, due to rapid urbanization, increasing healthcare expenditure, and expanding pharmaceutical manufacturing capabilities. There is new domestic drug manufacturing, exporting centers in such countries such as India, China, and South Korea, with government programs related to boosting pharmaceutical research and development in the host country, investing in infrastructure, and legal reforms. Emerging competitors like Dr. Reddy Laboratories, Sun Pharmaceutical Industries, and Samsung Biologics do help in capacity building and invention. Moreover, the increased consciousness towards chronic diseases and the increase in the number of patients makes it necessary to demand more sophisticated drug delivery forms, such as different coated tablets.
For instance, in November 2025, Sun Pharmaceutical Industries reported expansion of its oral solid dose manufacturing facilities in India, specifically increasing production capacity for coated tablets targeting respiratory, gastro-intestinal, and chronic disease segments to serve growing demand across the Asia Pacific coated tablets market.
Global Coated Tablets Market Outlook for Key Countries
How is the U.S. Helping in the Growth of the Coated Tablets Market?
The market of coated tablets in the U.S. remains on the lead in the world with high rates of technological implementation in the medicine production industry and well-developed system of research institutions. Such companies as Pfizer and Johnson and Johnson are concentrating on creation of innovative coated pills with higher effect and patient adherence. The FDA regulation factors are on quality and safety, which promotes advanced coating methods such as film and enteric coating methods. Additionally, the high amounts of healthcare spending and insurance coverage allow it to distribute it easily, which supports its good presence in the international market.
How is India Helping in the Growth of the Coated Tablets Market?
The India coated tablets market is growing at a high rate given the fact that it is a pharmacy of the world owing to large generic drug manufacturing plants that are run by firms like Sun Pharmaceutical and Dr. Reddy Laboratories. Government initiatives focused on increasing the pharmaceutical supply chain and enhancing regulatory quality promote the growth of the market. These characteristics of the middle class and the rise of healthcare awareness in the country lead to the increase of demand toward coated tablets where affordability and more developed systems of drug delivery are regarded as priorities.
Key Drivers for the Growth of the China Coated Tablets Market
China offers a promising market with a wide presence of government funding on healthcare infrastructures and pharmaceutical research. Significant companies such as Shanghai Pharma and CSPC Pharmaceutical Group dominate the marketplace through both the local supply and export markets. The programs like the Made in China 2025 program are encouraging pharmaceutical technological growth, such as some processes that enhance the stability of drugs and their bioavailability. The increasing prevalence of chronic diseases and the growing insurance coverage is an additional stimulus to coated tablets.
Germany Coated Tablets Market Trends
Germany will always be at the center of the coated tablets market in Europe because of the mature pharmaceutical industry that has stringent regulatory measures. Bayer and Merck KGaA companies are technological innovators concentrating on precision coating methods with consideration in specific therapeutic requirements. Excessive healthcare systems are enabled by efficient health care systems and the trade dynamics within the European Union ensure cross border distributions. The government subsidies pharmaceutical research and development are a guarantee of long-term competitiveness and quality improvement of coated tablets.
Region-wise Comparison of Generic vs Branded Coated Tablet Volume and Pricing Trends
|
Region |
Generic Coated Tablets – Volume Trend |
Generic Coated Tablets – Price Trend |
Branded Coated Tablets – Volume Trend |
Branded Coated Tablets – Price Trend |
|
North America |
High consumption driven by insurance-led generic substitution, growing chronic disease cases, and strong domestic manufacturing capacity supporting continuous demand growth. |
Strong pricing pressure due to pharmacy benefit managers, competitive supplier presence, and policy support for lower-cost generics. |
Stable to moderately growing volumes driven by oncology, specialty, and patented therapies requiring coated formulations. |
Premium pricing sustained for innovative therapies, though selective price negotiations and reimbursement pressures exist. |
|
Europe |
Strong growth due to government cost-containment policies encouraging generic drug adoption across public healthcare systems |
Prices decline gradually due to centralized procurement, tender systems, and reimbursement controls across EU countries |
Moderate volume growth in branded medicines, especially specialty and hospital-administered therapies |
Pricing controlled through strict reimbursement frameworks and price reference systems across member states |
|
Asia Pacific |
Rapid expansion supported by rising healthcare access, domestic pharmaceutical production growth, and increasing export-oriented generic manufacturing |
Highly competitive pricing environment due to large supplier base and price-sensitive domestic markets. |
Growing branded medicine demand in urban areas and private healthcare sectors driven by income growth and better healthcare access |
Prices remain moderate but rise gradually for innovative imported or specialty therapies |
|
Latin America |
Steady generic volume growth supported by government initiatives promoting affordable drug access and expansion of local manufacturing capabilities. |
Prices remain low due to public procurement programs and regulatory price controls in key markets. |
Branded drug volumes grow slowly in private healthcare and urban markets where insurance coverage is improving. |
Higher pricing persists compared to generics due to import reliance and limited domestic branded manufacturing. |
|
Middle East & Africa |
Gradual increase in generic drug consumption due to expansion of public healthcare programs and essential medicine access initiatives. |
Generic pricing kept low through government procurement contracts and subsidy mechanisms. |
Branded drug volumes remain dominant in private healthcare sectors and higher-income patient segments. |
Prices remain high due to heavy reliance on imported branded medicines and limited regional production capacity. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- On February 2, 2026, Piramal Pharma Solutions announced the successful development, scale-up, and commercialization of a tablet-in-capsule drug delivery system at its drug product facilities in Pithampur and Ahmedabad, India, enhancing its formulation and manufacturing offerings for oral therapies.
- On January 5, 2026, Cosmos Health Inc. announced that its subsidiary, Cana Laboratories, has entered into a manufacturing and supply agreement with Libytec Pharmaceutical Company for the production of the pharmaceutical product PathMuscle.
- In March 2025, Astellas Pharma Canada announced the nationwide availability of VEOZAH (fezolinetant film-coated tablets) across pharmacies in Canada, marking the launch of the country’s first non-hormonal NK3 receptor antagonist indicated for the treatment of moderate to severe vasomotor symptoms associated with menopause, commonly known as hot flashes.
Top Strategies Followed by Global Coated Tablets Market Players
|
Player Type |
Strategic Focus |
Examples |
|
Established Market Leaders |
Leading pharmaceutical companies maintain dominance in the coated tablets market by investing heavily in advanced formulation technologies, improving coating efficiency, and expanding global manufacturing and distribution partnerships to serve growing demand across both developed and emerging markets. |
Pfizer continues expanding global oral solid dosage production facilities to support coated tablet supply for chronic therapies across North America and emerging markets, strengthening both manufacturing scale and distribution reach. |
|
Mid-Level Players |
Mid-level pharmaceutical companies compete by offering cost-effective coated tablet solutions, leveraging partnerships and contract manufacturing collaborations to expand production capacity and reach price-sensitive markets without heavy R&D investments. |
Cipla partners with regional contract manufacturing firms to expand coated generic tablet production and supply affordable medicines across emerging markets in Asia Pacific and Africa. |
|
Small-Scale Players |
Small-scale manufacturers compete by focusing on niche coated tablet formulations, adopting efficient manufacturing technologies, and building regional partnerships to address specific therapeutic or patient needs in local markets. |
Regional pharmaceutical firms in Southeast Asia collaborate with local research institutes to develop taste-masked coated pediatric tablets tailored for domestic healthcare markets. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Report Scope
Coated Tablets Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.04 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.5% | 2033 Value Projection: | USD 1.96 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
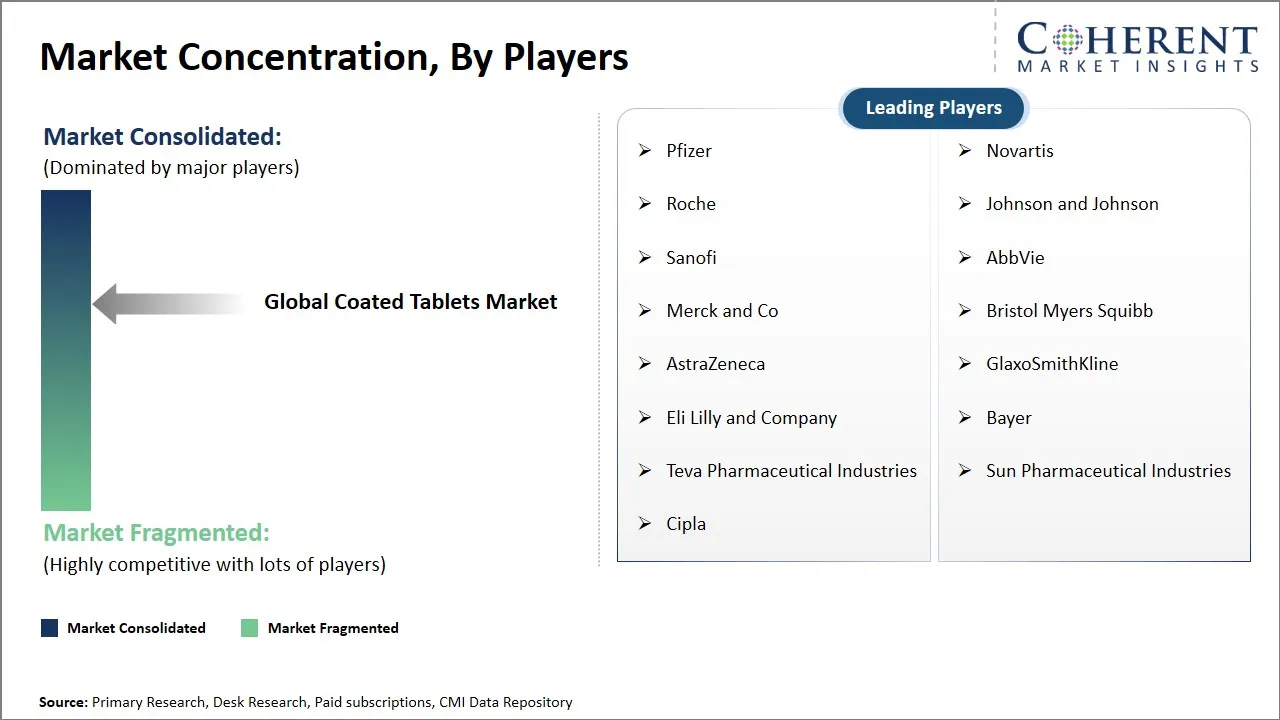
Pfizer, Novartis, Roche, Johnson and Johnson, Sanofi, AbbVie, Merck and Co, Bristol Myers Squibb, AstraZeneca, GlaxoSmithKline, Eli Lilly and Company, Bayer, Teva Pharmaceutical Industries, Sun Pharmaceutical Industries, and Cipla |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Coated Tablets Market Dynamics
To learn more about this report, Download Free Sample
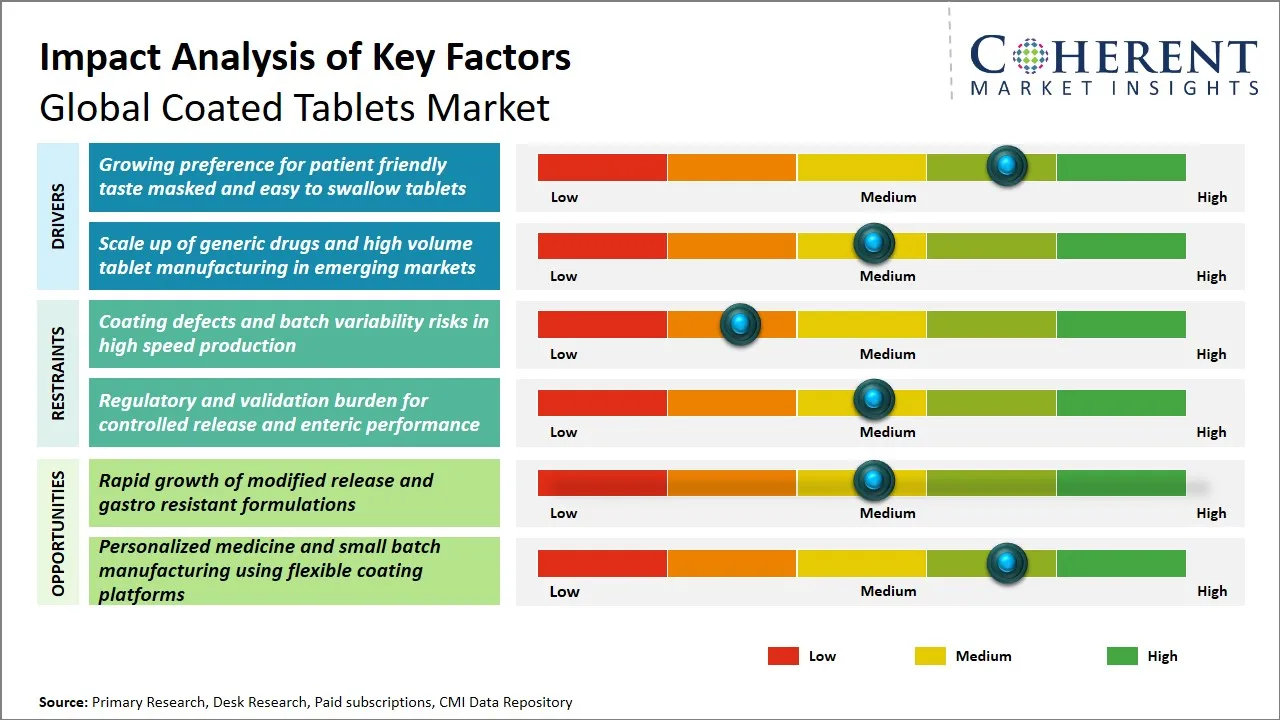
Global Coated Tablets Market Driver - Growing Preference for Patient Friendly Taste Masked and Easy to Swallow Tablets
One of the major forces that have been driving the market of coated tablets all over the world is the increased desire of patients and medical practitioners to use formulations that are more likely to promote drug compliance by improving palatability and ease of use. The use of coated tablets that are effective to cover unpleasant tastes is useful in overcoming one of the biggest obstacles to patient compliance especially among the pediatric and geriatric populations, which are more susceptible to bitter or unappealing flavors.
Also, challenges presented by people with dysphagia or the challenge to swallow standard oral dosage forms are handled by the development of coating that can make their swallowing of oral dosage easier. This patient-focused design is making drug producers invest in newer coating technologies that can enhance the sensory experience as well as safeguard active pharmaceutical molecules, ensuring their stability, and controlled release characteristics.
For instance, in September 2024, Haleon expanded distribution of its Panadol film-coated tablet range across multiple international markets, emphasizing faster swallowing and improved taste masking to enhance patient comfort and adherence, particularly among elderly and sensitive patient groups, reflecting rising demand for easy-to-consume coated oral tablets.
(Source- https://australasianpharmacy.com.au/articles/2024/10/haleon-set-to-roll-out-new-panadol-pack-sizes)
Global Coated Tablets Market Opportunity - Rapid Growth of Modified Release and Gastro-Resistant Formulations
The global coated tablets market is experiencing tremendous opportunities due to the swift increase in modified release and gastro resistant formulations. These new drug delivery technologies provide a better patient compliance profile due to the controlled drug release profiles, which reduce the dosage frequency and side effects. Extended-release and delayed-release systems are modified release surfaces that are used in chronic conditions like cardiovascular diseases, diabetes, and nervous disorders where a long-term treatment effect is essential.
Also, active pharmaceutical ingredients are covered by gastro-resistant coating to prevent degradation in the acidic stomach environment and allow target delivery in the intestines to improve drug bioavailability. The increase in gastrointestinal disorders and the ageing population is another area that increases the need of gastro-resistant tablet as it is less irritable and more effective in treatment.
For instance, in July 2024, AstraZeneca expanded availability of its modified-release oral oncology therapies in multiple European markets, leveraging advanced controlled-release tablet formulations to improve dosing convenience and maintain therapeutic drug levels, reflecting accelerating adoption of modified-release and gastro-resistant oral formulations in chronic disease treatment.
(Source- https://www.astrazeneca.com/media-centre/press-releases/2024/)
Analyst Opinion (Expert Opinion)
- The market of coated tablets is experiencing steady growth as pharmaceutical companies focus on patient-friendly oral dosage form of medication. This dosage form enhances stability, helps in taste masking and swallowing particularly in chronic treatment where extended drug compliance is necessary. Increasing generic drug production, growth of modified and controlled release formulations, and greater demand for gastro-resistant tablets are affecting the growth of the market.
- Advancement in technology in aqueous and polymer-based coatings are assisting manufacturers in complying with stricter environmental and regulatory requirements. Various growth prospects exist, including nutraceutical growth, customized dosing forms, and increased pharmaceutical usage in Asia Pacific and Latin America.
- The innovation of oral dosage and manufacturing efficiency has been a frequent topic of discussion in the industry with recent meetings like CPHI Worldwide, AAPS PharmSci 360 in the U.S., and Controlled Release Society annual meetings featuring companies and regulators discussing sustainable coating material, patient centric formulation design and next generation drug delivery technologies.
- The latest trends in the industry also show how investments and partnerships are reinforcing coated pill production worldwide. Novartis has added manufacturing capacity in the U.S. to accommodate oncology therapy, whereas Piramal Pharma Solutions has offered the ability to convert tablets into capsules to provide flexibility of dosing and altered release solution to international pharmaceutical companies.
- In Asia Pacific, generic drug manufacturers Sun Pharma and Cipla are still increasing production capacity of coated tablets to meet domestic and export needs. Simultaneously, regulatory authorities throughout North America and Europe are promoting safer and more patient-friendly dosage forms by expedited approvals, and lifecycle management revamps, making a positive climate to innovation in coated pill technology to ensure long term market expansion potential.
Market Segmentation
- Coating Type Insights (Revenue, USD Bn, 2021 - 2033)
- Film Coated Tablets
- Sugar Coated Tablets
- Enteric Coated Tablets
- Compression Coated Tablets
- Gelatin Coated Tablets
- Coating Material Insights (Revenue, USD Bn, 2021 - 2033)
- Polymer-Based Coatings
- Sugar-Based Coatings
- Cellulosic Coatings
- Acrylic-Based Coatings
- Natural Coatings
- Functional Objective Insights (Revenue, USD Bn, 2021 - 2033)
- Immediate Release Coating
- Delayed Release Coating
- Sustained / Controlled Release Coating
- Taste Masking Coating
- Moisture & Light Protection Coating
- Therapeutic Application Insights (Revenue, USD Bn, 2021 - 2033)
- Cardiovascular Drugs
- Gastrointestinal Drugs
- Anti-Infective Drugs
- CNS Drugs
- Pain Management Drugs
- Vitamins & Dietary Supplements
- Others (Respiratory, Anti-Diabetic, etc.)
- End User Insights (Revenue, USD Bn, 2021 - 2033)
- Pharmaceutical Manufacturers
- Nutraceutical Manufacturers
- Contract Manufacturing Organizations (CMOs)
- Distribution Channel Insights (Revenue, USD Bn, 2021 - 2033)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Institutional Sales
- Regional Insights (Revenue, USD Bn, 2021 - 2033)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Pfizer
- Novartis
- Roche
- Johnson and Johnson
- Sanofi
- AbbVie
- Merck and Co
- Bristol Myers Squibb
- AstraZeneca
- GlaxoSmithKline
- Eli Lilly and Company
- Bayer
- Teva Pharmaceutical Industries
- Sun Pharmaceutical Industries
- Cipla
Sources
Primary Research Interviews
Industry Stakeholders
- U.S. Food and Drug Administration officials
- European Medicines Agency regulatory experts
- Pharmaceutical manufacturing plant heads
- Drug formulation and coating technology specialists
- Contract manufacturing organization executives
- Pharmaceutical quality assurance and compliance managers
End Users
- Hospital pharmacy procurement heads
- Retail pharmacy chain purchasing managers
- Clinical pharmacologists
- Hospital formulary decision makers
- Chronic disease specialists prescribing oral tablets
- Healthcare procurement agencies
Government and International Databases
- U.S. Food and Drug Administration Drug Database
- European Medicines Agency Medicine Database
- World Health Organization Global Health Observatory
- OECD Health Statistics Database
- World Bank Health and Pharmaceutical Indicators
- UN Comtrade Pharmaceutical Trade Database
Trade Publications
- Pharmaceutical Technology Magazine
- European Pharmaceutical Review
- Pharma Manufacturing Magazine
- Drug Development and Delivery
- International Pharmaceutical Industry Magazine
- Pharmaceutical Processing World
Academic Journals
- International Journal of Pharmaceutics
- European Journal of Pharmaceutical Sciences
- Journal of Controlled Release
- AAPS PharmSciTech
- Drug Development and Industrial Pharmacy
- Pharmaceutical Research Journal
Reputable Newspapers
- The Wall Street Journal
- Financial Times
- The New York Times
- The Guardian
- The Washington Post
- The Economic Times
Industry Associations
- International Pharmaceutical Federation
- International Society for Pharmaceutical Engineering
- Controlled Release Society
- Pharmaceutical Research and Manufacturers of America
- European Federation of Pharmaceutical Industries and Associations
- Parenteral Drug Association
Public Domain Resources
- ClinicalTrials.gov
- WHO Medicines Technical Reports
- European Commission Public Health Reports
- National Institutes of Health Publications
- Centers for Disease Control and Prevention Publications
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients