Medical Exoskeleton Market Size and Forecast – 2025 to 2032
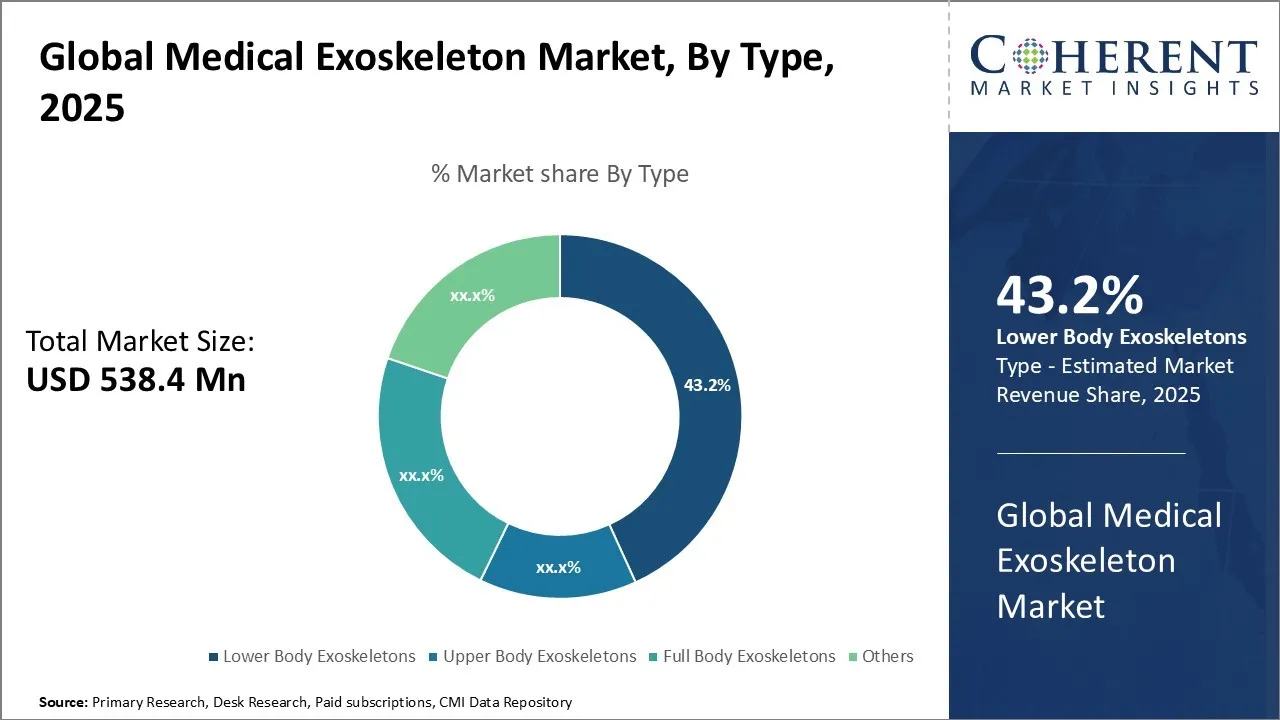
Global Medical Exoskeleton Market is estimated to be valued at USD 538.4 Mn in 2025 and is expected to reach USD 2,267.2 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of22.8% from 2025 to 2032.
Key Takeaways
- By Type, Lower Body Exoskeletons acquired the prominent share of 43.2% in 2025 on account of the rising incidence of mobility-impairing conditions.
- By Mobility, Mobile Exoskeleton dominates the overall market share in 2025 owing to the increasing demand for personal mobility assistance.
- By End User, Hospitals holds the largest market share in 2025 owing to its rising demand for advanced rehabilitation technologies.
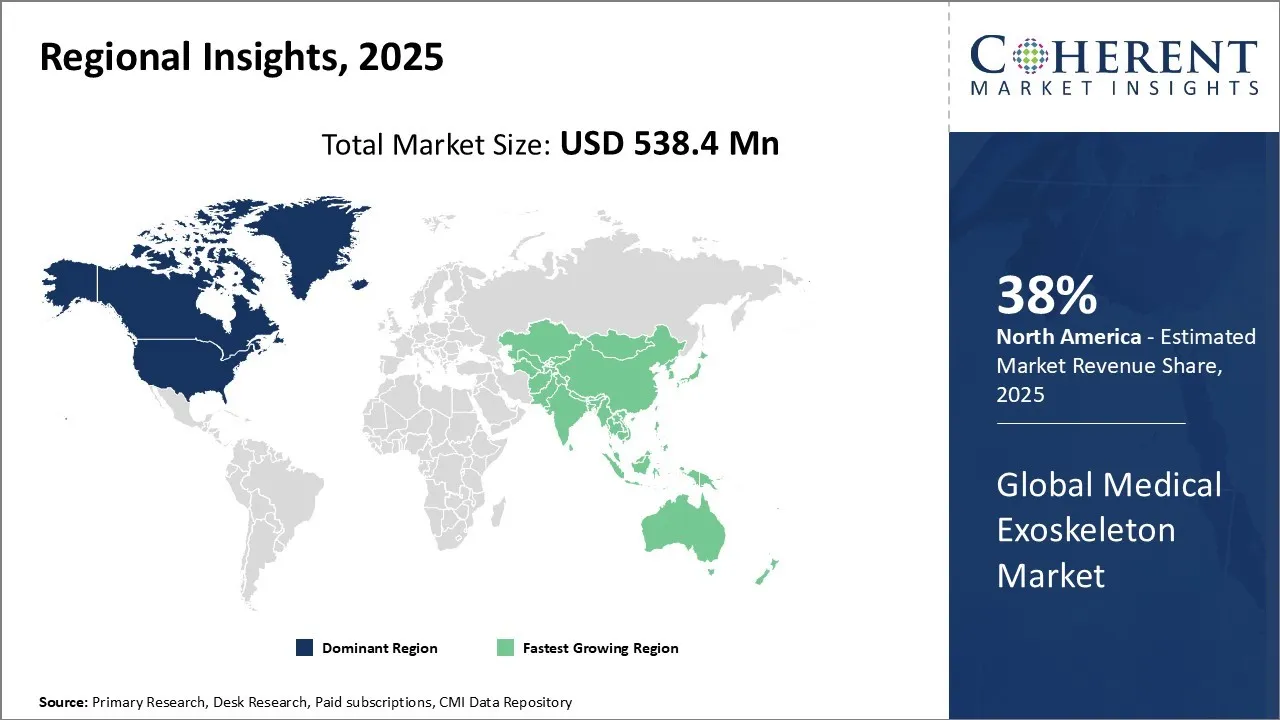
- By Region, North America dominates the overall market with an estimated share of 38% in 2025 owing to the high prevalence of neurological and mobility disorders across the United States.
Market Overview
The global medical exoskeleton market is segmented by type, mobility, end user, and region. Medical exoskeletons are wearable devices that work in tandem with the user’s movements to provide mobility assistance and improve strength. They enable individuals with mobility impairments or muscle weakness to stand, walk, lift heavier objects, and perform occupational and rehabilitation activities.
Current Events and their Impact on the Medical Exoskeleton Market
|
Current Events |
Description and its impact |
|
Technological Advancements in AI and Robotics |
|
|
Geopolitical and Trade Dynamics |
|
|
Clinical Research and Adoption Trends |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Role of Artificial Intelligence (AI) in Medical Exoskeleton
AI plays a transformative role in medical exoskeletons by enabling real-time adaptability, personalized assistance, and enhanced safety. It analyzes user movements to provide tailored support, making mobility more natural and efficient. Through predictive algorithms, AI anticipates motion and adjusts the device accordingly, improving user comfort and performance. It also monitors rehabilitation progress, offering valuable feedback to patients and clinicians.
In May 2025, German Bionic unveiled the Exia, its most advanced AI-powered exoskeleton system to date. Designed as an all-in-one solution, Exia combines enhanced strength, intelligence, and adaptability through fully integrated augmented AI. Developed using billions of real-world motion data points, the system features cutting-edge hardware architecture and refined control software for optimal performance.
End-user Feedback and Unmet Needs in the Medical Exoskeleton Market
- Comfort and Ergonomics Challenges: End-users often report discomfort during prolonged use due to bulkiness, weight, and fit issues. They seek lighter, more ergonomic designs that accommodate different body types and reduce fatigue. Addressing comfort remains a critical unmet need for wider acceptance in both clinical and daily use scenarios.
- Limited Battery Life and Portability: Users express frustration with short battery life restricting mobility and independence. Many mobile exoskeletons require frequent charging, limiting use outside clinical settings. Enhancing battery efficiency and overall portability is essential to meet patient demands for longer, uninterrupted usage in real-world environments.
- High Cost and Insurance Gaps: The high price of medical exoskeletons poses a significant barrier to adoption. Many users face limited insurance coverage or complex reimbursement processes, making these devices financially inaccessible. Improved insurance policies and cost reduction strategies are critical to expanding user access globally.
Medical Exoskeleton Market Insights, By Type: Lower Body Exoskeletons contribute the highest share of the market owing to its increasing adoption in hospitals and rehabilitation centers
Lower Body Exoskeletons acquired the prominent share of 43.2% in 2025. Rising cases of spinal cord injuries, stroke, and age-related mobility issues are driving the demand for lower body exoskeletons in the medical exoskeleton market. Hospitals and clinics increasingly adopt these devices to meet the growing need for advanced rehabilitation solutions. Continuous technological advancements have improved efficiency and usability, promoting wider clinical use. As awareness of their therapeutic benefits grows, supportive insurance policies and government initiatives further boost adoption. These exoskeletons actively help patients regain mobility, independence, and a better quality of life. For instance, in May 2025, Seoul introduced the high-tech “K-Smart Policing” pilot program in Yeouido, with the National Police Agency leading the initiative. The program features advanced tools like drones, electric bicycles, and wearable robots. As part of this effort, they introduced “WIM,” a robotic device designed to support lower body movement and reduce physical strain.
Medical Exoskeleton Market Insights, By Mobility: Mobile Exoskeletons contribute the highest share of the market owing to its shift toward outpatient and home-based rehabilitation
The growing need for personal mobility assistance among individuals with neurological disorders and physical impairments drives the demand for mobile exoskeletons in the medical exoskeleton market share. Manufacturers continue to improve usability through advancements in lightweight materials, battery life, and smart sensor technology, making these devices suitable beyond clinical environments. Healthcare providers increasingly adopt them for home-based rehabilitation and remote care. Rising awareness, expanded insurance support, and veteran healthcare programs further boost adoption. Mobile exoskeletons actively enhance patient mobility, independence, and overall quality of life.
For instance, in June 2025, Apptronik, the Texas-based company behind the Apollo humanoid, launched Elevate Robotics. As part of the launch, the company introduced a range of technologies, including exoskeletons, upper-body humanoid robots, bipedal mobility platforms, mobile manipulators, and robotic arms capable of lifting more than their own weight.
Medical Exoskeleton Market Insights, By End-User: Hospitals contribute the highest share of the market owing to its pressure to improve operational efficiency
Hospitals actively drive growth in the medical exoskeleton market by adopting advanced technologies to enhance patient care and rehabilitation outcomes. They implement exoskeletons to aid recovery for patients with stroke, spinal cord injuries, and other mobility impairments. These devices ease the physical burden on therapists, improve treatment efficiency, and accelerate patient recovery. Advancing technology and increasing clinical evidence encourage hospitals to integrate exoskeletons into therapy programs. Supportive reimbursement policies and government funding further motivate hospitals to invest in rehabilitation robot solutions. For instance, German Bionic introduced the Apogee+ exoskeleton to support healthcare workers by using electrically powered robotic motors to assist their muscles. When nurses and other medical staff wear the Apogee+, they can lift heavier patients than usual. The motorized suit also enables them to move patients more easily, with less strain and greater endurance during long hospital shifts.
Regional Insights
To learn more about this report, Download Free Sample
North America Medical Exoskeleton Market Trends
North America dominates the overall market with an estimated share of 38% in 2025. Increasing investments in healthcare infrastructure and technological innovation shape the North American medical exoskeleton market. Strong government support, favorable reimbursement policies, and a growing aging population needing mobility assistance benefit the region. Leading companies and research institutions actively drive product development and clinical adoption. Hospitals, rehabilitation centers, and veteran healthcare programs implement exoskeletons to improve patient outcomes. Additionally, rising awareness of robotic rehabilitation benefits fuels demand, establishing North America as a key market for medical exoskeleton advancements. For instance, in April 2025, KULR Technology Group, Inc., a leader in advanced energy management platforms announced a new strategic partnership with German Bionic (GB), a global robotics company renowned for its innovative robotic exoskeleton, Apogee ULTRA. Through this collaboration, both companies aim to expand into the rapidly growing robotics and artificial intelligence sectors.
Asia Pacific Medical Exoskeleton Market Trends
Asia Pacific countries are heavily investing in healthcare infrastructure by expanding hospitals and rehabilitation centers. This expansion drives greater demand for advanced medical technologies like exoskeletons, which support mobility rehabilitation and patient care, especially in emerging economies such as India and China. Local hospitals and international companies collaborate to provide training programs, helping users become familiar with exoskeleton technology and encouraging wider clinical acceptance. The availability of cutting-edge exoskeletons attracts international patients, boosting the market as they seek quality post-injury and neurological care. For instance, Royal Rehab, an Australian healthcare provider, has signed an agreement with U.S.-based robotics company Ekso Bionics to introduce advanced exoskeleton technology in Australia. This partnership supports Royal Rehab’s goal to become a center of excellence in technology-driven rehabilitation by acquiring assistive technologies that help patients move more freely, communicate, control objects, and perform daily tasks.
United States Medical Exoskeleton Market Trends
The Veterans Affairs healthcare system in the U.S. strongly adopts exoskeletons, providing extensive support and funding for wounded veterans. This drives demand for rehabilitation-focused exoskeletons and establishes the U.S. as a leader in veteran-specific medical exoskeleton use. Medicare pilot programs and private insurers expand patient access and actively encourage clinical adoption, setting the U.S. market apart. For instance, in April 2025, Lifeward Ltd., a global leader in innovative medical technology for individuals with physical limitations or disabilities, has announced the national launch of the ReWalk 7 Personal Exoskeleton in the United States. This marks the debut of the next generation of the company’s groundbreaking personal exoskeleton technology.
Japan Medical Exoskeleton Market Trends
Japanese companies collaborate closely with universities and research institutions to advance exoskeleton technologies. This partnership drives innovation in lightweight materials, ergonomics, and user-friendly controls, producing high-quality, culturally adapted medical devices tailored to Japanese healthcare settings. Japan leverages its global expertise in robotics and precision manufacturing to create sophisticated medical exoskeletons. Companies integrate advanced AI and sensor technologies into their products, enhancing patient outcomes and strengthening market competitiveness. For instance, Japanese Company Cyberdyne has released the latest version of its HAL exoskeleton. Designed primarily for the healthcare sector, HAL—short for Hybrid Assistive Limb—continues to use bio-electrical signals from the wearer to operate. The new model, HAL Lumbar, builds on previous versions with similar signal-reading capabilities focused on supporting the lower back.
Market Report Scope
Medical Exoskeleton Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 538.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 22.8% | 2032 Value Projection: | USD 2,267.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Ekso Bionics, ReWalk Robotics, PARKER HANNIFIN CORP, BIONIK, Rex Bionics Ltd, Suit X, CYBERDYNE INC., B-Temia, Wandercraft and ExoAtlet |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Medical Exoskeleton Market Trend
- Shift Toward Portable and Wearable Designs
Medical exoskeletons are evolving from bulky, stationary devices to lightweight, portable models that patients can use outside clinical settings. This shift supports home-based rehabilitation and daily mobility, empowering users with greater independence. Advanced materials, improved battery technology, and compact actuators enhance wearability and ease of use, differentiating newer devices from earlier models focused primarily on hospital or rehab center applications.
In November 2024, KAIST launched the WalkON Suit F1, a wearable robot designed by Professor Kyoungchul Kong and his team for individuals with complete disabilities. Unlike traditional exoskeletons, it can be worn directly from a wheelchair without assistance, marking a major advancement in mobility and independence.
Medical Exoskeleton Market Opportunity
- Home-Based Rehabilitation Expansion
The growing demand for home-based rehabilitation presents a significant opportunity for medical exoskeletons. As healthcare shifts towards patient-centric care, portable and easy-to-use exoskeletons enable continuous therapy outside clinical settings. This not only improves patient convenience and recovery outcomes but also reduces healthcare costs by minimizing hospital stays and therapy sessions. Companies that develop user-friendly, remote-monitoring capable devices can tap into this expanding market segment.
Medical Exoskeleton Market News
- In February 2025, Wandercraft launched a landmark clinical trial for its Personal Exoskeleton technology. Designed to restore upright movement and walking independence, the Personal Exoskeleton targets individuals with severe mobility impairments, aiming to significantly improve their daily mobility and quality of life.
- In July 2025, Hippos Exoskeleton unveiled a knee brace equipped with airbags designed to prevent ACL injuries. The company has begun accepting pre-orders for the upcoming consumer product and is already attracting interest from top sports organizations, including UK Athletics, Crystal Palace’s academy, and the Chinese Olympic Association.
Analyst Opinion (Expert Opinion)
- The medical exoskeleton market is poised for transformative growth driven by unprecedented technological convergence and shifting clinical paradigms. The true inflection point lies not merely in device innovation but in systemic integration within healthcare workflows. For example, Ekso Bionics’ partnership with the U.S. Department of Veterans Affairs demonstrates a crucial model where reimbursement and clinical validation align, overcoming two of the most persistent barriers to adoption. Their EksoNR device's demonstrated ability to reduce therapy time by up to 30% is not just a marketing claim but a quantifiable impact that reshapes rehabilitation protocols.
- Moreover, AI integration as a game-changer. ReWalk Robotics’ recent deployment of adaptive gait algorithms shows clear evidence of improved patient-specific outcomes, shifting exoskeletons from static assistive devices to intelligent, responsive therapeutic platforms. This level of personalization is critical for addressing diverse patient profiles, particularly in conditions like multiple sclerosis where symptoms fluctuate.
- In Asia Pacific, aggressive government funding and regulatory encouragement, especially in Japan and South Korea, indicate a strategic recognition of exoskeletons as essential eldercare technology, not just rehabilitation tools. This contrasts with Western markets, which still largely view exoskeletons through a narrow post-injury lens. Companies that tailor their product lines to Asia’s unique demographic pressures stand to dominate the global landscape.
Market Segmentation
- By Type
- Lower Body Exoskeletons
- Upper Body Exoskeletons
- Full Body Exoskeletons
- Others (Soft Exoskeletons, Active Exoskeletons, Passive Exoskeletons)
- By Mobility
- Mobile Exoskeletons
- Stationary Exoskeletons
- Fixed Exoskeletons
- By End User
- Rehabilitation Centers
- Hospitals
- Long Term Care Centers
- Homecare Settings
- Others (Industrial Sites, Military)
- By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Ekso Bionics
- ReWalk Robotics
- PARKER HANNIFIN CORP.
- BIONIK
- Rex Bionics Ltd.
- Suit X
- CYBERDYNE INC.
- B-Temia
- Wandercraft
- ExoAtlet
Sources
Primary Research Interviews
- Healthcare professionals and rehabilitation therapists
- Medical device manufacturers and engineers
- Hospital procurement managers
- Patients using medical exoskeletons
- Government health officials
Databases
- PubMed
- ClinicalTrials.gov
- WHO Global Health Observatory
- FDA Medical Device Databases
- National Institutes of Health (NIH) databases
Magazines
- IEEE Spectrum
- MedTech Insight
- Rehabilitation Times
- Robotics Business Review
- HealthTech Magazine
Journals
- Journal of NeuroEngineering and Rehabilitation
- Archives of Physical Medicine and Rehabilitation
- Journal of Rehabilitation Medicine
- Robotics and Autonomous Systems
- IEEE Transactions on Neural Systems and Rehabilitation Engineering
Newspapers
- The New York Times Health Section
- The Guardian Health News
- The Wall Street Journal Health & Science
- Japan Times (Health Section)
- South China Morning Post Health
Associations
- American Physical Therapy Association (APTA)
- International Society of Physical and Rehabilitation Medicine (ISPRM)
- Robotics Industries Association (RIA)
- European Society of Physical and Rehabilitation Medicine (ESPRM)
- Japan Society of Medical and Biological Engineering
Public Domain Sources
- U.S. Department of Veterans Affairs reports
- WHO reports on aging and mobility
- National Health Service (NHS) rehabilitation guidelines
- Government health policy publications (Japan, South Korea, India)
- Open-access clinical trial results
Proprietary Elements
- CMI Data Analytics Tool, and Proprietary CMI Existing Repository of information for last 8 years
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients