Global Nicotine Replacement Therapy Market Size and Share Analysis - Growth Trends And Forecasts (2025-2032)
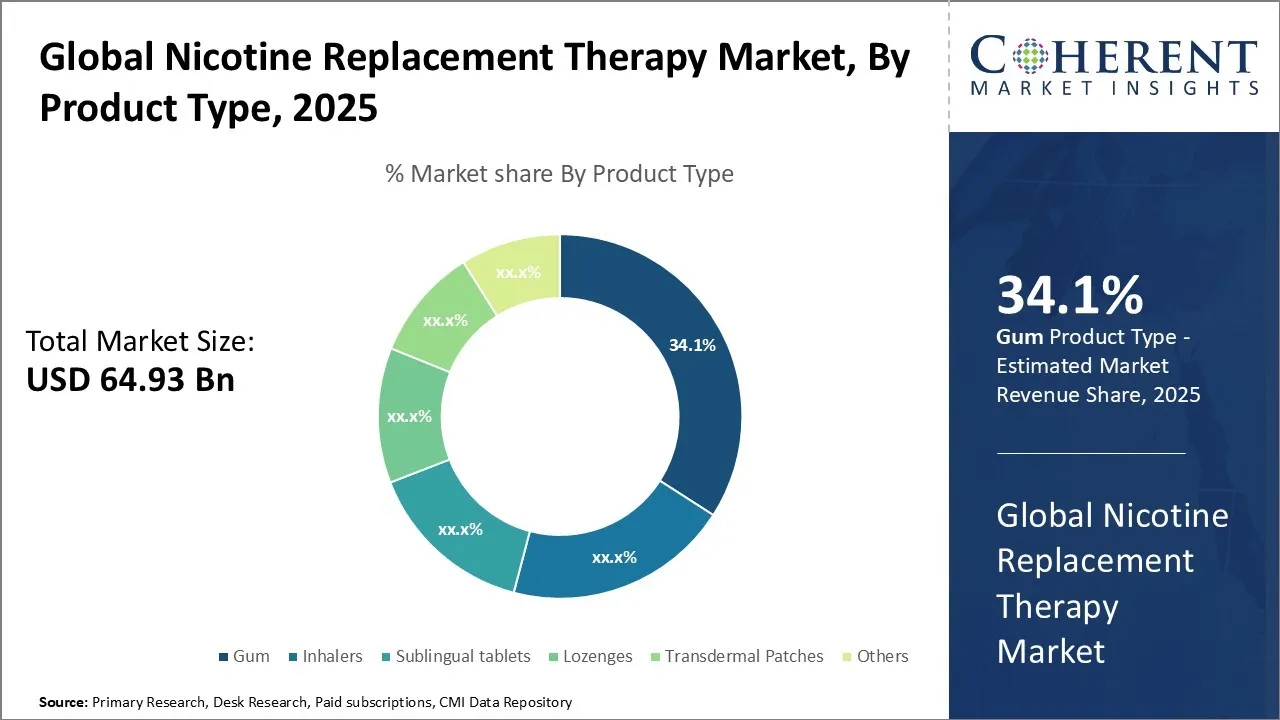
Global Nicotine Replacement Therapy Market is estimated to be valued at USD 64.93 Bn in 2025 and is expected to reach USD 128.15 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 10.2% from 2025 to 2032.
Key Takeaways
- By Product Type, the Gum segment is projected to dominate the global nicotine replacement therapy market with a 34.1% share in 2025, underscoring its popularity as a convenient and fast-acting cessation aid.
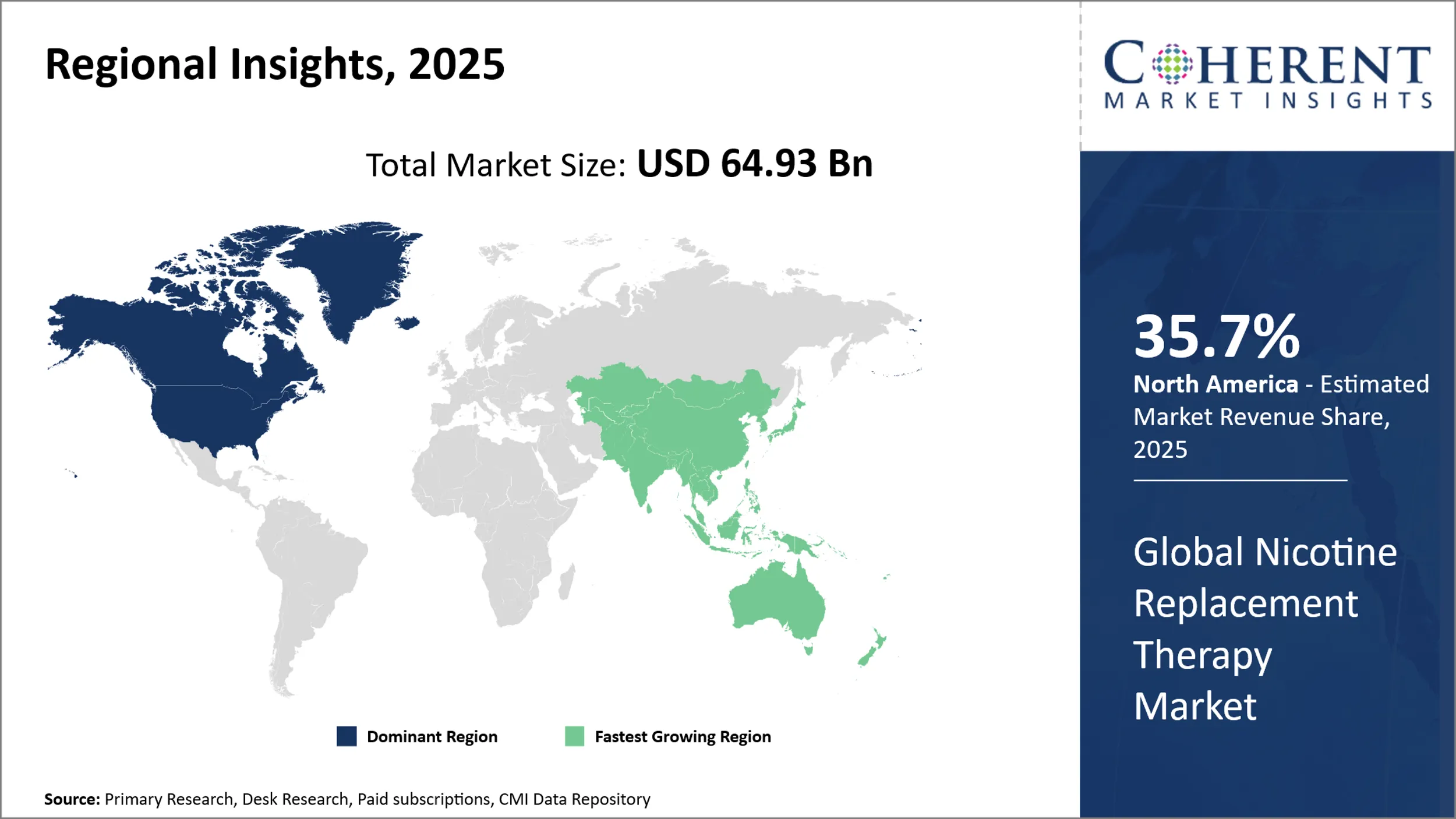
- North America is anticipated to lead the global nicotine replacement therapy market with a 35.7% share in 2025, driven by increasing smoking cessation efforts and strong regulatory support.
- Asia Pacific is poised for around 25.2% shares in the global market during the forecast period, owing to rising health awareness and rapid economic growth.
- Europe is projected to hold about 19% shares in the global market in 2025, due to rising prevalence of smoking cigarettes in the region.
Market Overview
The global Nicotine Replacement Therapy (NRT) Market is witnessing consistent growth, driven by increasing awareness about the health risks of smoking and growing efforts to quit tobacco use. NRT products like gums, patches, and lozenges offer safer alternatives to nicotine intake, helping users manage withdrawal symptoms. The gum segment leads due to its convenience and fast action.
Current Events and its Impact on the Nicotine Replacement Therapy Market
|
Current Event |
Description and its impact |
|
Regulatory Push for Smoking Cessation Programs |
|
|
Supply Chain Volatility and Raw Material Costs |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement Scenario: Nicotine Replacement Therapy Market
The reimbursement landscape for nicotine replacement therapy (NRT) varies significantly across regions and plays a crucial role in driving market adoption. In developed markets such as the United States, United Kingdom, Germany, and Canada, many NRT products—including patches, gums, lozenges, and prescription-based therapies—are covered under public health insurance schemes or employer-sponsored plans.
For instance, the U.S. Affordable Care Act mandates coverage of smoking cessation treatments, including NRT, without co-payments in many plans. Similarly, the UK's National Health Service (NHS) offers subsidized or free access to NRT as part of national cessation programs. However, in many developing countries, reimbursement remains limited or non-existent, restricting access to these therapies for low-income populations.
As health systems worldwide recognize the long-term cost savings of reducing smoking-related illnesses, there is a growing push to include NRT in essential medicines lists and reimbursement schemes. Improved reimbursement policies are expected to enhance product affordability, accessibility, and overall market penetration.
Nicotine Replacement Therapy Market Trends
- Rising awareness about health risks
As awareness about the various health risks associated with smoking has continued to increase across the globe, it has prompted many individuals to try and quit smoking. Studies have shown that smoking cigarettes increases the risk of various cancer types as well as other chronic diseases such as heart disease and stroke.
The negative health impacts of smoking have been widely publicized through anti-smoking campaigns by governments and health organizations. This has led to a change in social attitudes towards smoking over the past few decades. More people now see cigarettes as a health risk rather than a casual pleasure. As a result, the desire to quit smoking has grown significantly.
In May 2025, Business Standard reported that Cipla Health launched Nicotex Begin, a mobile app integrating a 12-week personalized smoking cessation program with WHO-recommended NRT protocols (gum, patches, lozenges). The app is positioned to quintuple quit success rates, reflecting innovation in digital support for the NRT market.
- Stress of modern lifestyle
In today's fast-paced world with changing societal pressures and personal challenges, stress levels seem to be rising across populations. Juggling responsibilities at home and work, information overload, financial demands are some common stress triggers for urban dwellers. Stress management has become an important part of self-care. However, unhealthy coping mechanisms like smoking continue to be popular.
The temporary stress relief from nicotine makes it difficult for many smokers to quit, especially during stressful periods. With stressful modern lifestyles now prevalent across both developed and developing nations, cigarette smoking persists partly due to its role in relieving stress.
In June 2024, Reuters reported that Haleon, the maker of Nicotinell gum, patches, and lozenges, agreed to sell its nicotine replacement therapy business outside the U.S. to India’s Dr. Reddy’s for £500 million (~USD 633 million). This divestment underscores consolidation and strategic repositioning in the global NRT market.
Opportunities in the Nicotine Replacement Therapy Market
- Combination NRT therapy
Combination Nicotine Replacement Therapy (NRT) refers to the use of more than one type of NRT product concurrently to improve the chances of successfully quitting smoking. Research has shown that using a combination of NRT products can be more effective than using a single form of NRT. In the global NRT market, the following points highlight the importance and impact of combination NRT therapy:
Increased Efficacy: Using two forms of NRT together, such as nicotine gum and patches, often increases the overall level of nicotine, which can more effectively manage withdrawal symptoms and cravings, leading to higher success rates in smoking cessation.
Targeted Relief: A combination approach allows for both continuous nicotine delivery (through patches) and the ability to address sudden cravings (with short-acting forms like gum, lozenges, or inhalers).
Market Differentiation: Products that are specifically designed to be used in combination offer a unique selling point and can create market differentiation for manufacturers.
Patient-Centered Care: Combination NRT can be personalized to individual needs, making it a flexible option for healthcare providers to recommend based on the specific habits and preferences of their patients.
Rising Healthcare Expenditure
One of the significant factors influencing the growth rate of the global nicotine replacement therapy market is the growing healthcare expenditure, which helps in improving its infrastructure. For instance, according to the International Health Care System of the U.S., in June 2020, U.S. government organizations aim to improve the healthcare infrastructure by increasing funding, setting legislation and national strategies, and cofounding and setting basic requirements and regulations for the Medicaid program.
Similarly, in November 2022, the Canadian Institute for Health Information reported that the total health spending in Canada was USD 331 billion in 2022, or USD 8,563 per Canadian, while health expenditure represented 12.2% of Canada's gross domestic product (GDP) in 2022, following a high of 13.8% in 2020.
Nicotine Replacement Therapy Market Insights, By Product Type
The gum segment is projected to dominate the global nicotine replacement therapy market with a 34.1% share in 2025, underscoring its popularity as a convenient and fast-acting cessation aid. This leadership is driven by its ability to deliver nicotine without smoking, making it a preferred choice for individuals seeking discreet and controlled dosing.
The segment benefits from over-the-counter availability, ease of use, and its appeal among users who need flexible usage throughout the day. Gum’s fast relief from cravings, combined with strong support from healthcare professionals and public health campaigns, further boosts its adoption. While other forms like patches and lozenges are gaining traction, gum continues to lead the market due to its portability, immediacy of effect, and widespread consumer familiarity.
Regional Insights
To learn more about this report, Download Free Sample
North America Nicotine Replacement Therapy Market Trends and Analysis
North America is anticipated to lead the global nicotine replacement therapy market, accounting for a substantial 35.7% share in 2025. This leadership is fuelled by increasing smoking cessation efforts, strong regulatory backing, and rising public awareness of tobacco-related health risks. Government-led initiatives, such as higher tobacco taxes and insurance-covered cessation programs, have significantly boosted NRT adoption across the U.S. and Canada.
The region’s robust healthcare infrastructure, coupled with the widespread availability of NRT products through retail pharmacies and e-health platforms, supports high consumer engagement. Additionally, the growing use of digital health solutions and online pharmacies is enhancing accessibility and convenience, further strengthening North America’s dominance in the market.
Europe Nicotine Replacement Therapy Market Trends and Analysis
Europe remains a prominent player in the global nicotine replacement therapy market, supported by long-standing public health policies and high awareness of the dangers of smoking. Countries like the UK, Germany, and France actively promote cessation through national health campaigns and widespread OTC availability of NRT products.
The presence of established pharmaceutical companies and consistent regulatory support ensures continued product innovation and accessibility. As European consumers increasingly seek smoke-free alternatives, the market benefits from a mature demand base and government-funded initiatives, solidifying the region’s strong position.
Nicotine Replacement Therapy Market Dominating Countries
United States and Canada
The United States is a dominant force in the nicotine replacement therapy market, driven by strong public health initiatives, rising tobacco taxes, and widespread insurance coverage for cessation treatments. The country benefits from a robust healthcare infrastructure and high consumer awareness, with significant usage of gums, patches, and lozenges supported through both retail and digital health platforms. Continuous innovation and strong OTC availability further enhance its market position.
Canada also plays a vital role in regional growth, with its universal healthcare system supporting access to cessation therapies. National smoking reduction strategies and growing health consciousness are accelerating the adoption of NRT products, especially among younger and urban populations.
Market Report Scope
Nicotine Replacement Therapy Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 64.93 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.2% | 2032 Value Projection: | USD 128.15 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Johnson & Johnson Inc. , Haleon Group of Companies, Imperial Brands plc, Cipla Inc., Pfizer Inc., GLENMARK PHARMACEUTICALS LTD, Fertin Pharma, Philip Morris Products S.A., British American Tobacco plc, Japan Tobacco Inc., Habitrol., Perrigo Company plc, Die betapharm Arzneimittel GmbH, ITC Limited , Sparsha Pharma International Pvt Ltd, and Alkalon A/S |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Analyst Opinion ( Expert Opinion)
- The global nicotine replacement therapy (NRT) is experiencing growth due to growing awareness about the health risks of smoking and strong government initiatives promoting cessation. They highlight the growing adoption of NRT products such as gums, patches, and lozenges, driven by consumer demand for safer and more convenient alternatives to smoking. The gum segment, in particular, is viewed as a consistent market leader due to its ease of use and over-the-counter availability.
- Experts point to a rising trend in innovation, with companies focusing on novel delivery formats like oral sprays, nicotine pouches, and smart patches. There is also increased emphasis on synthetic, tobacco-free nicotine formulations to meet evolving regulatory requirements and consumer preferences.
- Regionally, North America leads the market with robust healthcare support and high public awareness, while Asia Pacific, especially India and China, is emerging as a high-growth region due to expanding healthcare access and digital distribution channels.
Nicotine Replacement Therapy Market: Key Development
- In June 2025, The FDA began scientific review of Swedish Match’s modified-risk tobacco product (MRTP) applications for the 20 authorized Zyn pouches, potentially allowing “reduced-risk” marketing claims upon approval.
- In February 2025, the Punjab & Haryana High Court ruled that NRT products (gum, patches, and inhalers) can be sold in retail outlets, removing them from India’s Poisons Act 1919 list. This decision greatly increases access to NRT across the region, making it more readily available to smokers seeking cessation aids. Experts view this shift as pivotal in promoting quitting through improved accessibility, ultimately supporting public health objectives.
- In January 2025, the U.S. FDA granted marketing authorization for 20 Zyn nicotine pouch products via the PMTA pathway the first such approval for nicotine pouches in the country. This breakthrough allows adult smokers to access a tobacco-free, lower-risk alternative to cigarettes and traditional smokeless tobacco. It also signals regulatory acceptance of innovative delivery formats and fuels investment in nicotine pouch manufacturing by Philip Morris International.
- In November 2024, the U.S. FDA approved an Investigational New Drug (IND) application for Qnovia's RespiRx Nicotine Inhaler (QN-01) the first inhalable NRT device designed for fast pulmonary nicotine delivery. This approval opens the door for clinical trials comparing its pharmacokinetics and efficacy against existing inhaler therapies and cigarettes. By potentially offering quicker craving relief, QN-01 could enhance cessation success and introduce a new, prescription-based inhaler format to the NRT landscape.
- In October 2024, British American Tobacco announced plans to launch Velo Plus, its synthetic nicotine pouch, in the U.S. in 2025 marking its entry into the tobacco-free nicotine space.
Market Segmentation
- Global Nicotine Replacement Therapy Market, By Product Type
- Inhalers
- Gum
- Transdermal Patches
- Sublingual Tablets
- Lozenges
- Others
- Global Nicotine Replacement Therapy Market, By Distribution Channel
- Online
- Offline
- Global Nicotine Replacement Therapy Market, By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Johnson & Johnson Inc.
- Haleon Group of Companies
- Imperial Brands plc.
- Cipla Inc.
- Pfizer Inc.
- GLENMARK PHARMACEUTICALS LTD.
- Fertin Pharma
- Philip Morris Products S.A.
- British American Tobacco plc.
- Japan Tobacco Inc.
- Perrigo Company plc.
- Die betapharm Arzneimittel GmbH
- ITC Limited
- Sparsha Pharma International Pvt Ltd.
- Alkalon A/S
Sources
The Stakeholders Consulted
- Pharmacists and clinical practitioners specializing in smoking cessation
- Manufacturers and suppliers of nicotine replacement therapy products (gums, patches, lozenges, sprays, inhalers)
- Tobacco control experts and public health consultants
- Regulatory bodies overseeing OTC and prescription drug approvals
- Health insurers and pharmacy benefit managers
- Research institutions focused on addiction medicine and behavioral health
- Hospital procurement managers and retail pharmacy chains
- End-users including adult smokers, ex-smokers, and behavioral therapy groups
Databases Opened
- U.S. Food and Drug Administration (FDA) – Drug Approvals and Tobacco Control Resources
- European Medicines Agency (EMA) – Nicotine Replacement Therapy Registries
- WHO Global Health Observatory – Tobacco Use and Cessation Program Data
- Centers for Disease Control and Prevention (CDC) – Tobacco Use & Prevention Database
- National Health Service (UK) – Stop Smoking Services Data
Magazines & Trade Publications
- Pharmaceutical Technology
- Drug Store News
- Chain Drug Review
- Tobacco Reporter – Harm Reduction Section
- The Pharma Letter – Smoking Cessation Market News
- Medical News Today – Smoking and Addiction Treatments
Scientific and Industry Journals
- Nicotine & Tobacco Research
- Journal of Smoking Cessation
- Addiction
- BMJ – Tobacco Control
- The Lancet Public Health
- Journal of Substance Abuse Treatment
Newspapers & Media Outlets
- The Wall Street Journal – Health & Pharma
- Bloomberg – Healthcare and Regulatory News
- Reuters – Pharmaceutical Industry Updates
- The Economic Times – Healthcare & Life Sciences
- Business Standard – Public Health & OTC Market Trends
Associations and Regulatory Bodies
- World Health Organization (WHO) – Framework Convention on Tobacco Control (FCTC)
- U.S. Food and Drug Administration (FDA) – Center for Tobacco Products
- National Institute for Health and Care Excellence (NICE), UK
- Centers for Disease Control and Prevention (CDC)
- Indian Ministry of Health & Family Welfare – National Tobacco Control Programme
- European Network for Smoking and Tobacco Prevention (ENSP)
Public Domain Sources
- WHO Tobacco Free Initiative Reports
- U.S. CDC Office on Smoking and Health Publications
- National Cancer Institute (NCI) – Smoking Cessation Resources
- Eurostat – Tobacco Product Sales and Usage Statistics
- OECD – Public Health & Smoking Behavior Metrics
- UK Office for National Statistics – Smoking Prevalence & Quit Success Rates
Proprietary Research Elements
- CMI Data Analytics Tool
- Proprietary CMI Repository of Market Data (covering past 8 years)
- CMI Expert Interviews and Transcripts (focused on NRT usage trends, behavioral insights, and sales performance across regions)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients