Global Xgeva Market Size and Forecast – 2025 to 2032
The Global Xgeva Market is estimated to be valued at USD 2,501.1 Million in 2025 and is expected to reach USD 3,140.7 Million by 2032, exhibiting a compound annual growth rate (CAGR) of 2.7% from 2025 to 2032. This steady growth reflects increasing adoption of Xgeva in treating bone-related conditions and metastatic cancers, driven by expanding patient populations and enhancements in therapeutic protocols.
Key Takeaways of the Global Xgeva Market:
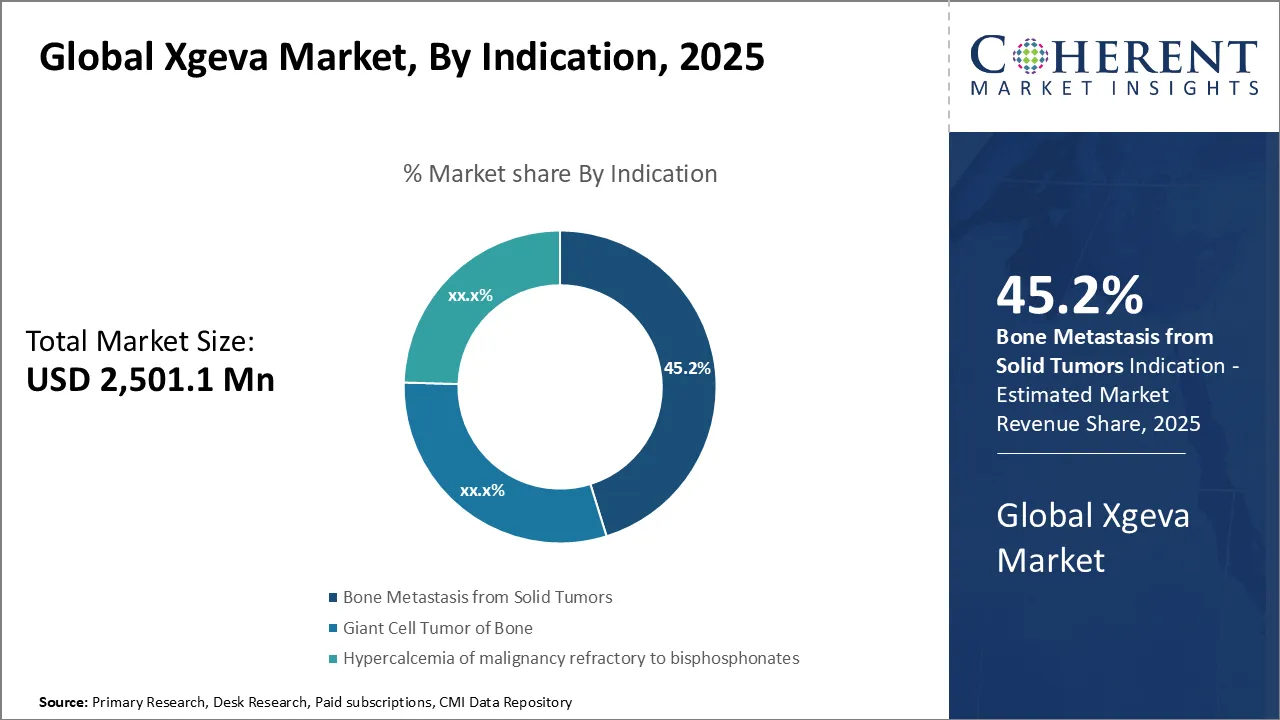
- In 2025, the bone metastasis from solid tumors segment is expected to dominate the global Xgeva market with a 45. 2% share.
- The Adult segment is projected to hold the largest share by age group at 31. 2% in 2025.
- In terms of sales channels, the online segment leads with an estimated 53. 2% share in 2025.
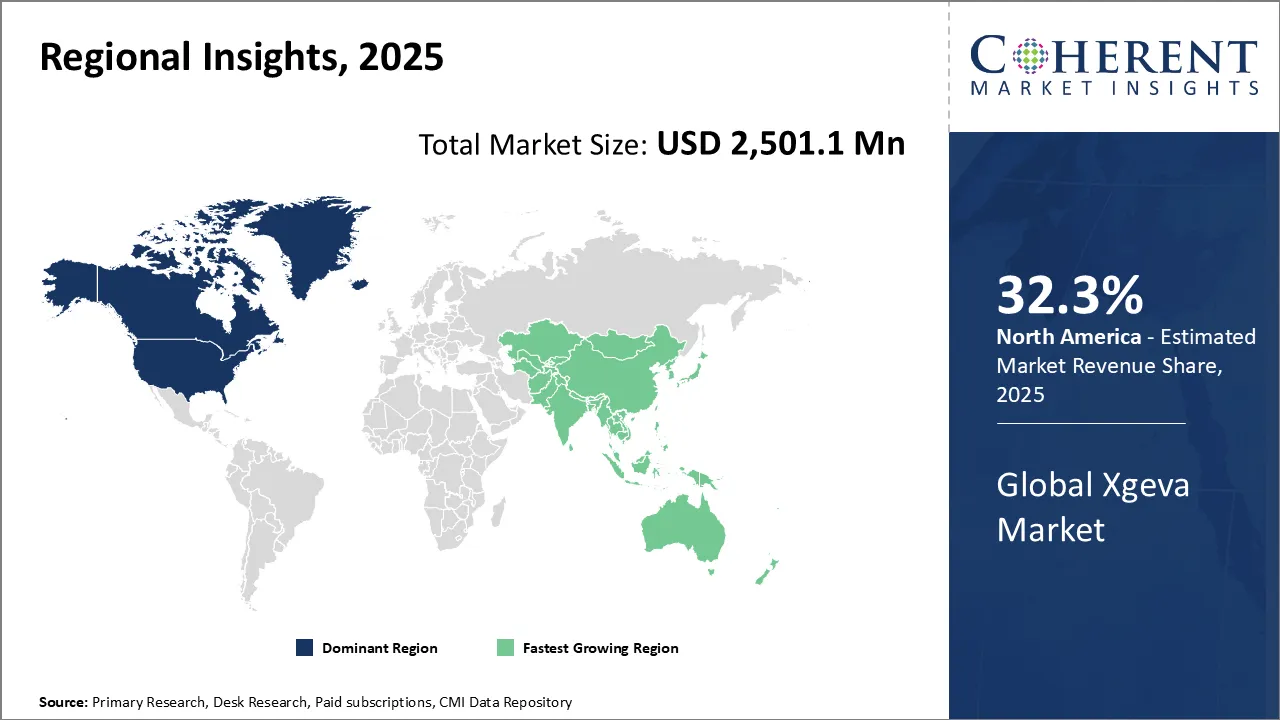
- North America is expected to lead the market, holding a share of 32. 3% in 2025.
- Asia Pacific is anticipated to be the fastest-growing region, with a market share of 23. 4% in 2025.
Market Overview:
A key market trend is the rising focus on personalized medicine and the integration of Xgeva with other cancer treatments to improve patient outcomes. Additionally, growing awareness about bone metastasis management and ongoing clinical research for new indications are fueling demand. Market players are also investing in developing biosimilars to increase affordability and accessibility, further shaping the competitive landscape and sustaining market expansion.
Currents Events and their Impact
|
Current Events |
Description and its impact |
|
USFDA Approves Multiple Biosimilars for Xgeva |
|
|
Regulatory Approvals in Europe |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Xgeva Market Insights, By Indication - Bone Metastasis from Solid Tumors Dominates Due to High Prevalence and Complexity of Bone Metastases
In terms of indication, the Bone Metastasis from Solid Tumors segment is expected to command the largest share of the global Xgeva market with 45.2% in 2025, primarily fueled by the high prevalence and complexity of bone metastases in various cancers. Bone metastasis is a common and severe complication observed in patients with solid tumors such as breast, lung, and metastatic prostate cancer. These metastases often lead to debilitating skeletal-related events (SREs), including pathological fractures, spinal cord compression, and severe pain, prompting urgent medical intervention. Xgeva has emerged as a critical therapeutic option due to its mechanism of action in inhibiting RANKL, which plays a pivotal role in osteoclast-mediated bone destruction. This results in the preservation of bone integrity and significant prevention of SREs among affected patients.
The growing incidence of cancers associated with bone metastases directly stimulates demand for Xgeva, especially in oncology care centers where managing complications related to bone health is a priority. Improvements in cancer survival rates also mean that more patients live longer with bone metastases, extending the need for effective bone-targeting treatments. Moreover, the preference for Xgeva over traditional bisphosphonates is rising, largely because of its superior efficacy in reducing skeletal complications and a comparatively favorable safety profile in patients with renal impairment. This has encouraged oncologists to increasingly recommend Xgeva as a frontline therapy for bone metastasis management.
Global Xgeva Market Insights, By Age Group - Adult Remains Dominant Due to Higher Cancer Incidence and Treatment Accessibility
The adult segment holds the highest share of the global Xgeva market, with a share of 31.2% in 2025 in terms of age group, which can be attributed to the higher incidence of cancers linked with bone complications among this population. Adults, typically defined as individuals in the age bracket from approximately 18 to 65 years, represent the core demographic frequently diagnosed with types of solid tumors that metastasize to bone, such as breast and prostate cancers. This age group is more likely to undergo aggressive cancer treatments, including chemotherapy and hormone therapies, which can exacerbate bone weakening and increase the risk of skeletal-related events, thus driving the demand for preventative treatments like Xgeva.
Additionally, adults are generally more proactive in seeking medical care and adhering to prescribed treatment regimens, supported by better health insurance coverage and healthcare access compared to pediatric or geriatric populations in many regions. This facilitates steady uptake of Xgeva in adult patients to manage bone complications effectively. The adult population also benefits significantly from emerging clinical guidelines emphasizing the critical role of bone-targeted therapy in maintaining quality of life during cancer treatment, which has translated into increased prescriptions of Xgeva.
Global Xgeva Market Insights, By Sales Channel - Online Leads Due to Convenience and Expanding Digital Healthcare Ecosystem
From the perspective of sales channels, the online segment holds the highest share in the global Xgeva market with a share of 53.2% in 2025, reflecting broader shifts towards digital healthcare ecosystems and changing consumer purchasing behaviors. Increasing digital penetration and the rising adoption of e-pharmacies play a significant role in driving online sales. Patients and healthcare providers find online platforms convenient for ordering prescription medications like Xgeva, which may require timely replenishment to manage ongoing conditions such as bone metastases or giant cell tumors effectively.
Online sales channels offer discrete, round-the-clock access to medications that patients with severe or chronic conditions often need, coupled with home delivery services that enhance convenience and adherence. Especially in the post-pandemic era, the adoption of telemedicine and online pharmaceutical services accelerated dramatically, helping overcome barriers related to geographical access and reducing the burden on brick-and-mortar healthcare facilities. More importantly, online channels facilitate seamless communication between patients, healthcare providers, and pharmacies, streamlining the ordering and fulfillment process for complex therapies like Xgeva.
Pricing Analysis of Xgeva Market
- The pricing of Xgeva varies significantly across different countries, with the United States maintaining the highest price of approximately USD 3,692.67 for a 1.7 ml vial in 2024. In contrast, countries like Saudi Arabia, Canada, and India offer the drug at much lower prices, with India pricing it at USD 335.32. This discrepancy is largely due to differences in healthcare systems, regulatory environments, and market competition. The drug's cost is influenced by complex production processes, patent protection, and the role of insurance coverage, which can substantially impact out-of-pocket expenses for patients. Insurance schemes in countries like the U.S. and Canada play a key role in determining affordability, often making the drug more accessible, though sometimes at a high cost for patients without comprehensive coverage.
- Looking ahead, the pricing of Xgeva is expected to decrease by up to 60% by 2032, driven by the increasing availability of biosimilars and heightened market competition. As multiple Food and Drug Administration (FDA)-approved biosimilars enter the market, their competition is anticipated to drive prices lower, especially in countries with high healthcare costs like the U.S. and Canada, where price changes of 25%-30% are forecasted. In contrast, countries with stricter price controls, such as France and Germany, may see more modest price reductions of around 15%. Regulatory policies, biosimilar adoption, and the ongoing demand for Xgeva’s multiple indications will be key factors shaping the drug's future pricing trajectory.
Regional Insights:
To learn more about this report, Download Free Sample
North America Xgeva Market Analysis and Trends
North America Xgeva market dominates driven largely by a well-established healthcare infrastructure and strong pharmaceutical industry presence, with a share of 32.3% in 2025. The region benefits from advanced clinical trial networks and early adoption of innovative cancer therapies, facilitating faster patient access to drugs like Xgeva. Government policies, including robust patent protections and favorable regulatory frameworks from agencies such as the USFDA, further bolster market stability and growth. The presence of leading biopharmaceutical companies, particularly Amgen, the developer of Xgeva, strengthens the market ecosystem. Moreover, comprehensive insurance coverage and rising healthcare expenditures, driven by the increasing incidence of cancer and growing healthcare investments, enhance patient access to medication in both the U.S. and Canada.
Asia Pacific Xgeva Market Analysis and Trends
The Asia Pacific region exhibits the fastest growth for the Xgeva market, fueled by increasing cancer incidence and growing healthcare investments, with a share of 23.4% in 2025. Rapid improvements in healthcare infrastructure, expanding clinical research activities, and increasing patient awareness are key drivers of this uptrend. Government initiatives aimed at enhancing oncology care, alongside gradual improvements in regulatory pathways, support quicker market penetration. Countries such as Japan, China, and Australia are witnessing heightened demand for novel cancer treatments. Multinational pharmaceutical companies are actively forming strategic partnerships and local alliances to navigate this diverse and rapidly evolving market landscape, facilitating accelerated adoption of Xgeva.
In the Asia Pacific region, governments are prioritizing oncology care through strategic policies and funding. For instance, in February 2025, India has allocated significant resources in the Union Budget 2025-26, with plans to establish Day Care Cancer Centers in district hospitals and provide customs duty exemptions for lifesaving cancer medications. Similarly, other countries in the region are focusing on expanding access to cancer care, improving early detection, and supporting cancer research, aiming to reduce the growing burden of cancer across the region.
Xgeva Market Outlook for Key Countries
U.S. Xgeva Market Trends
The U.S. is the leading market for Xgeva, driven by strong R&D investments and regulatory support. Amgen plays a key role in innovation, while FDA approvals expand Xgeva’s use in cancer care. Bone metastases are prevalent in U.S. patients with solid tumors, especially breast, lung, and prostate cancers, with 4.6% of patients developing bone metastasis within one year and 8.2% within 10 years. This high prevalence drives the demand for Xgeva to manage bone-related complications in cancer patients.
According to an American Society of Clinical Oncology (ASCO)study, using electronic medical records from 52 U.S. cancer centers found that among 382,733 patients, the incidence of bone metastasis was 4.6% at 1 year, 6.7% at 5 years, and 8.2% at 10 years. The study highlighted that bone metastasis incidence varies significantly by tumor type and stage at diagnosis, with prostate cancer showing the highest incidence (71.1% at 10 years for stage IV). The data provides more accurate real-world estimates, which could improve national prevalence figures, especially considering recent advances in cancer treatment.
Japan Xgeva Market Trends
Japan's market demonstrates strategic importance due to its aging population and high prevalence of bone-related complications in cancer patients. The presence of leading domestic pharma firms collaborating with Amgen enhances market reach. Japan’s government promotes innovative cancer treatments with expedited approval processes under programs like the Sakigake Designation System, contributing to faster Xgeva introduction. Oncology specialists’ focus on improving the quality of life for patients boosts prescription rates.
For instance, in July 2023, National Library of Medicine provided a data or published a data according to which Cancer is the leading cause of death among children, adolescents, and young adults (AYAs) in Japan, though it is rare. From 2016–2018, the cancer incidence was 166.6 per million for children (0–14 years) and 579.0 for AYAs (15–39 years). The types of cancer varied by age, with hematological cancers and CNS tumors common in children, bone tumors and sarcomas in teenagers, and carcinomas in young adults. Treatment was mostly provided at pediatric cancer hospitals (PCHs) for children, but less so for AYAs, highlighting the need for specialized cancer care systems for each age group.
China Xgeva Market Trends
China’s expanding oncology healthcare landscape is a significant factor in the growing Xgeva market. Government policies aimed at improving access to innovative therapies, such as the inclusion of drugs in national reimbursement lists, support patient affordability. International players, including Amgen, have entered into local partnerships to streamline regulatory approvals and distribution networks, enhancing market penetration.
India Xgeva Market Trends
The Xgeva market in India is expanding, driven by the development of biosimilars like Alvotech’s AVT03 and regulatory approvals for treating skeletal-related events in cancer patients. Clinical trials, such as Virchow Biotech’s Phase III study on its denosumab biosimilar, are paving the way for broader market access. With increasing global competition from biosimilars, the Indian market is set for growth, improving affordability and accessibility for patients in need of oncology and bone health treatments.
In September 2024, Ministry of External Affairs, Government of India, the United States, Australia, India, and Japan have launched the Quad Cancer Moonshot Initiative to tackle cancer in the Indo-Pacific region, starting with cervical cancer. The initiative aims to strengthen cancer care by improving health infrastructure, expanding research, and supporting cancer prevention, detection, treatment, and care.
Market Players, Key Developments, and Competitive Intelligence:

To learn more about this report, Download Free Sample
Key Developments:
- In February 2024, Amgen, one of the global biotechnology companies, announced that the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) had granted marketing authorization for its high-concentration Xgeva (denosumab) 120mg solution for injection in a prefilled syringe. This product, a line extension of the original Xgeva vial, is the first to be approved under the MHRA’s new International Recognition Procedure (IRP), which accelerates assessments by recognizing approvals from several major regions, including the EU and the U.S. The approval follows a positive opinion from the European Medicines Agency in January 2024.
- BeOne Medicines, Ltd, formerly known as BeiGene, Ltd., announced that China’s National Healthcare Security Administration (NHSA) has updated the National Reimbursement Drug List (NRDL) to include four new indications for tislelizumab, marking expanded access to this PD-1 inhibitor. The updates, effective from March 1, 2023, will improve access to these treatments in China.
- In November 2020, BeiGene, a commercial-stage biotechnology company focused on developing and commercializing innovative medicines, announced that the China National Medical Products Administration (NMPA) approved XGEVA (denosumab) for the prevention of skeletal-related events (SREs) in patients with bone metastases from solid tumors and multiple myeloma (MM). Under a strategic collaboration with Amgen, BeiGene began commercializing XGEVA in China in July 2020. The approval follows clinical trials involving over 7,000 patients, which showed that XGEVA significantly delayed the time to the first SRE compared to zoledronic acid in patients with bone metastases from various cancers, including breast cancer, castration-resistant prostate cancer (CRPC), and non-small cell lung cancer.
Market Report Scope
Global Xgeva Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,272.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 2.7% | 2032 Value Projection: | USD 3,140.7 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., BeOne Medicines, Ltd, formerly known as BeiGene, Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Xgeva Market Dynamics
To learn more about this report, Download Free Sample
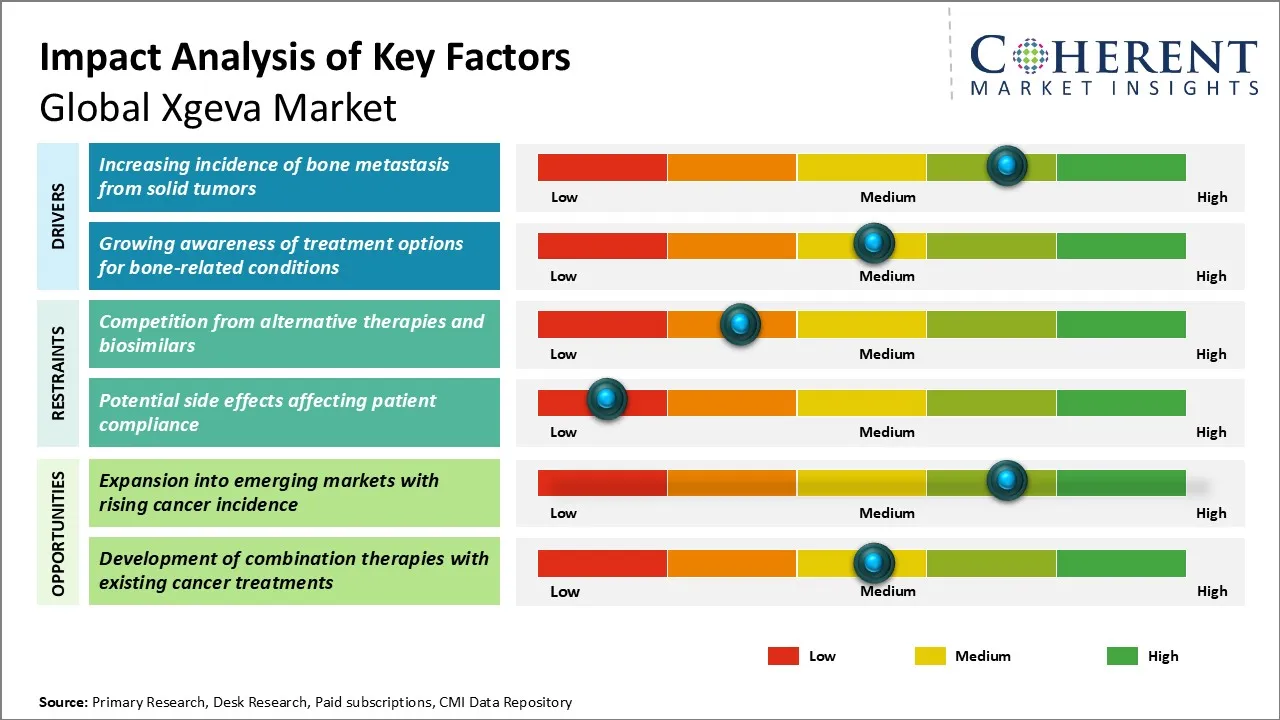
Xgeva Market Driver - Increasing Incidence of Bone Metastasis from Solid Tumors
The increasing incidence of bone metastasis among patients suffering from solid tumors such as breast, prostate, and lung cancers is a significant factor driving demand for Xgeva globally. Bone metastasis often leads to severe complications like skeletal-related events (SREs), including fractures, spinal cord compression, and the need for radiation or surgery to the bone, which substantially deteriorate patient quality of life and increase healthcare burdens. Xgeva, being an effective RANKL inhibitor, plays a crucial role in preventing these SREs by inhibiting osteoclast-mediated bone destruction. As the global population ages and cancer prevalence rises, the number of patients experiencing bone metastases is expected to grow. This trend intensifies the reliance on therapeutic agents like Xgeva that can manage skeletal complications and improve clinical outcomes.
In May 2024, a study by the National Library of Medicine using the Surveillance, Epidemiology, and End Results (SEER) database found that 5.2% of patients with solid tumors had bone metastases. The most common cancers associated with bone metastases were lung, prostate, breast, kidney, and colon cancers. Risk factors included Gleason score and prostate-specific antigen (PSA) for prostate cancer, HER2 and hormonal receptor status for breast cancer, histology for lung cancer, and liver metastases for colorectal tumors. Identifying these factors can help in early intervention.
Xgeva Market Opportunity: Expansion into Emerging Markets with Rising Cancer Incidence
The Global Xgeva market presents a significant growth opportunity through strategic expansion into emerging markets characterized by a rising incidence of cancer. Countries in regions such as Asia-Pacific, Latin America, and parts of Africa are experiencing an increasing cancer burden driven by factors including changing lifestyles, urbanization, and improved diagnostic capabilities. These markets remain underpenetrated due to limited access to advanced oncology therapies, creating a substantial unmet medical need. By leveraging Xgeva’s proven efficacy in preventing skeletal-related events in patients with bone metastases, pharmaceutical companies can tap into these growing patient populations.
In February 2024, according to World Health Organisation (WHO), the global cancer burden is growing rapidly, especially in emerging markets, with projections indicating a 77% increase in new cancer cases by 2050. This rise is primarily driven by aging populations, increasing exposure to risk factors like tobacco, alcohol, and obesity, and environmental issues such as air pollution. High-income countries will see the greatest absolute increase in cancer cases, but low and medium-income countries will experience the most significant proportional rise, with cancer incidence and mortality nearly doubling. This surge presents a significant opportunity for companies to expand cancer care services and treatments, particularly in countries with low and medium Human Development Index (HDI), where cancer care access and infrastructure are still limited. The expansion into these markets is essential for addressing both the growing demand and the existing healthcare disparities.
Analyst Opinion (Expert Opinion)
- The global Xgeva market is experiencing substantial growth, driven primarily by technological advancements in oncology drugs, increasing regulatory support, and the rising demand for targeted therapies in bone metastasis and other cancer-related conditions. The ongoing development of novel administration techniques and expanding approval for diverse oncology indications are fueling market expansion. Furthermore, emerging opportunities in personalized medicine, combined with the rising global awareness of bone-related disorders, are expected to propel the market further. However, challenges such as high treatment costs, the need for continuous research, and access issues in developing regions remain significant hurdles.
- In recent years, key conferences like the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) have significantly contributed to shaping the Xgeva market by discussing the latest advancements in cancer treatments, including bone metastasis therapies. These events serve as crucial platforms for knowledge exchange and the formulation of clinical guidelines, directly influencing the market’s direction. Case studies, such as the collaboration between Amgen and various research institutions, highlight the continued effort in expanding Xgeva’s use beyond its initial indications, positioning the drug as a key player in oncology care.
Market Segmentation
- Indication Insights (Revenue, USD Mn, 2020 - 2032)
-
- Bone Metastasis from Solid Tumors
- Giant Cell Tumor of Bone
- Hypercalcemia of malignancy refractory to bisphosphonates
- Age Group Insights (Revenue, USD Mn, 2020 - 2032)
-
- Adult
- Geriatric
- Adolescents
- Sales Channel Insights (Revenue, USD Mn, 2020 - 2032)
-
- Online
- Offline
- End User Insights (Revenue, USD Mn, 2020 - 2032)
-
- Hospitals
- Oncology Centers
- Rehabilitation Centers
- Others (Long-Term Care Facilities, etc.)
- Regional Insights (Revenue, USD Mn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Amgen Inc.
- BeOne Medicines, Ltd, formerly known as BeiGene, Ltd.
Sources
Primary Research Interviews
- Interviews with healthcare professionals (oncologists, orthopedic surgeons, rheumatologists)
- Interviews with key opinion leaders in the pharmaceutical industry
- Patient surveys and feedback from healthcare providers
- Discussions with healthcare institutions
Government and International Databases
- U.S. Food and Drug Administration (FDA)
- European Medicines Agency (EMA)
- World Health Organization (WHO)
- National Institutes of Health (NIH)
- Centers for Disease Control and Prevention (CDC)
- International Agency for Research on Cancer (IARC)
- U.S. National Library of Medicine (PubMed)
Trade Publications
- Pharmaceutical Executive
- BioPharma Dive
- Oncology Times
- Drug Discovery & Development
- Pharmaceutical Technology
Academic Journals
- Journal of Clinical Oncology
- The Lancet Oncology
- Osteoporosis International
- Journal of Bone and Mineral Research
Reputable Newspapers
- The New York Times
- The Wall Street Journal
- Financial Times
- The Guardian
Industry Associations
- American Society of Clinical Oncology (ASCO)
- American College of Rheumatology (ACR)
- International Osteoporosis Foundation (IOF)
- National Osteoporosis Foundation (NOF)
- European Society for Medical Oncology (ESMO)
Public Domain Resources
- U.S. National Library of Medicine (ClinicalTrials.gov)
- PubMed Central
- World Health Organization (WHO) Publications
- European Medicines Agency (EMA) Public Information
- National Cancer Institute (NCI)
Proprietary Elements:
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in the market.
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients