Asia Pacific Breast Implants Market is estimated to be valued at USD 439.9 Mn in 2025 and is expected to reach USD 790.8 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.74% from 2025 to 2032. Increasing prevalence of the breast cancer in the Asia Pacific region is expected to drive the market growth over the forecast period for the Asia Pacific region. For instance, in September 2022, according to the data published by the National Center for Biotechnology Information, it was stated that India has the highest breast cancer mortality rates, which was found to be 12.7 per 100,000 cases. Furthermore, according to the Centers for Disease Control and Prevention, it was found that the cancer incidence in Asia Pacific was 169.1 per 1,00,000 in the year 2021, accounting for 49.3% of the global cancer incidence.
Analysts’ Views on Asia Pacific Breast Implants Market :
The Asia Pacific breat implants market growth can be driven by an increasing approval of the breast implants. For instance, in January 2022, Mentor Worldwide LLC, subsidiary of the Johnson & Johnson, medical devices company, announced that the MENTOR MemoryGel BOOST breast implant got the approval from the U.S. Food and Drug Administration for breast augmentation in women at least 22 years old, and for women of all ages undergoing breast reconstruction.
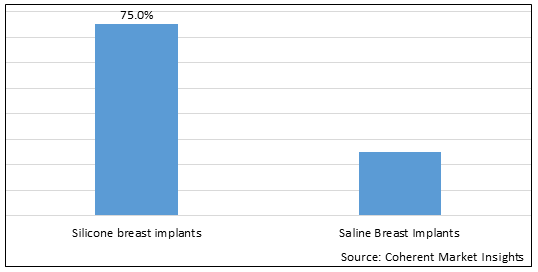
Figure 1. Asia Pacific Breast Implants Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Asia Pacific Breast Implants Market - Driver
Increasing clinical trial study to evaluate safety and efficacy of the breast implants to drive the Asia Pacific breast implants market over the forecast period
Th major market player are focused on carrying out clinical trials for launching new breast implants in the market. For instance, in June 2022, BellaSeno Pty Ltd, medical technology company, an Australia-based company, initiated a clinical trial for surgical implantation of the PCL breast scaffold with autologous fat grafting. The clinical trial study is estimated to get completed by June, 2026.
Asia Pacific Breast Implants Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 439.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.74% | 2032 Value Projection: | USD 790.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Allergan, Plc. (AbbVie Inc.), Mentor Worldwide LLC, GC Aesthetics Plc, Sientra, Inc., POLYTECH Health and Aesthetics GmBH, Establishment Labs S.A., Laboratories Arion, HanSBiomed Co. Ltd., Groupe Sebbin SAS, and GG Biotechnology Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing launch of the next generation of breast implants by the key players operating in the market
Key market players are adopting growth strategies such as launching next generation of breast implants in the market, which is expected to drive the Asia Pacific breast implants market over the forecast period. For instance, in May 2021, GC Aesthetics, Inc., medical technology company, announced the launched of the Perle, a next generation of breast implant. Perle is an innovative line of smooth breast implants that characterize a proprietary surface technology (BioQ) GC Aesthetics, Inc. company’s industry-leading gel technology (Emunomic Breast Tissue Dynamic Gel), and an enhanced version of the safety features.
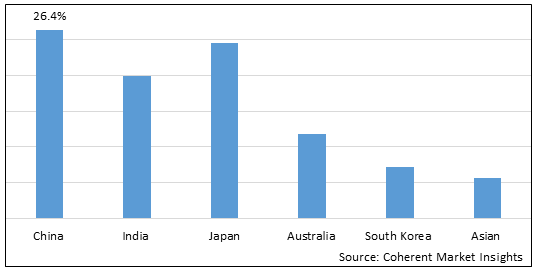
Figure 2. Asia Pacific Breast Implants Market Share (%), by Country, 2025
To learn more about this report, Download Free Sample
Asia Pacific Breast Implants Market - Country Analysis
Among all countries, China is expected to dominate the Asia Pacific market over the forecast period. This is attributed to China holding a XX.X% market share and an increasing prevelance of the breast cancer in China. For instance, in December 2025, according to the data published by the National Center for Biotechnology Information, it was estimated that 124,002 deaths and 2.79 million cases of the breast of cancer were reported in China in the year 2022.
Asia Pacific Breast Implants Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020.
The COVID-19 has affected the economy in three main ways: by directly affecting the production and demand for drugs and vaccines, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, UAE, Egypt, and others faced problems with regard to the transportation of drugs and vaccines from one place to another.
Quarantine, traveling constraints, and social distancing measures are likely to lead to a steep decline in business and consumer spending. Furthermore, healthcare providers were facing challenges in terms of additional manpower, equipment, consumables, and other resources, which were required to ensure safety in hospitals and provide treatment to patients with other diseases. This has impacted the overall healthcare sector negatively. Additionally, there were delays in hospitalization as the number of patient visits decreased, which is expected to affect the Asia Pacific breat implants market over the forecast period.
Asia Pacific Breast Implants Market Segmentation:
The Asia Pacific breat implants market report is segmented into product type, shape, application and end user.
Based on Product Type, the market is segmented into Silicone Breast Implants and Saline Breast Implants. Out of which, the Silicone Breast Implants segment is expected to dominate the Asia Pacific breast implants market during the forecast period and this is attributed to an increasing adoption of inorganic growth strategies such as collaboration to initiate the research and development activities for evaluating the safety and efficacy of new silicone breast implants. For instance, in November 2025, Seoul National University Hospital, a chain of hospitals in South Korea, in collaboration with HansBiomed Co.,Ltd., biotechnology company, initiated a clinical trial study to evaluate the efficacy and safety of breast implants. The aim of the clinical trial study was to evaluate the safety and efficacy of Cohesive Silicone Gel-Filled Breast Implant (CoSBI) produced by HansBiomed co.,Ltd. in breast reconstruction or augmentation. The study is estimated to get completed by November 2023.
Based on Shape, the market is segmented into Round Breast Implants and Anatomical Breast Implants. Out of which, the Round Breast Implants segment is expected to dominate the Asia Pacific breast implants market during the forecast period and this is attributed their efficiency to shift and rotate freely without disorting the shape of the breast. They are symmetrical in shape and eliminate any problem arising at the time of implant rotation. Moreover, round breast implants are touted to provide a rounder appearance and more lift to breast while visibly and significantly increasing their size. Therefore, even physician and surgeons prefer round – shaped breast implants over anatomical breast implants.
However, anatomical – shaped breast implants have certain limitations. The anatomical – shaped breast implants tend to change the shape of the entire organ, during any rotation or shift of the implant, which could lead to an additional surgery for recorrection.
Based on Application, the market is segmented into cosmetic surgery and reconstructive surgery. Out of which, the cosmetic surgery segment is expected to dominate the Asia Pacific breast implants market during the forecast period and this is attributed to increasing focus on enhancing aesthetic appeal among women. In September 2020, according to the article published by the Springer Nature, it was estimated that several studies have demonstrated that breast implants can help in boosting self-esteem, body image, and sexual satisfaction. Majority of the women are conscious about physical appearance of their breast, which may lead to high demand for cosmetic surgery for breast augmentation.
Based on End User, the market is segmented into Hospitals and Clinics. Out of which, the hospitals segment is expected to dominate the market over the forecast period and this is attributed to increasing prevalence of the breast cancer. For instance, in December 2022, according to the data published by the Cancer Australia, it was estimated that there were 3,214 deaths due to cancer in Australia among which about 34% deaths were due to breast cancer in the old age womens (65 years old -75 years old) in the year 2022.
Among all segmentation, the product type segment has the highest potential due to an increasing approval of new products by the regulatory bodies. For instance, in July 2022, Sientra, Inc., a medical aesthetic company, announced that it has received the U.S. Food and Drug Administration approval for the low plus profile projection breast augmentation in women at least 22 years old, and for women of all ages undergoing breast reconstruction.
Asia Pacific Breast Implants Market Cross Sectional Analysis:
In the product type segment, silicone breast implant segment held a dominant position in the China due to the increasing number of research and development activity for the cosmetic surgeries for breast enlargement. For instance, in February 2020, Allergan Plc., pharmaceutical company, initiated a clinical trial study to evaluate the safety of McGhan single lumen gel-filled breast implants in patients in China. In this study, a retrospective chart analysis, analyzes the medical files of patients who underwent breast augmentation or reconstruction using McGhan silicone breast implants between December 24, 2017 and December 31, 2020, and who were enrolled in about 5 hospitals or clinics in China.
In application segment, cosmetic surgery held a dominant position in the Hopitals due to increasing research and development activity carried out by the hospitals for evaluation of the breast implants used in cosmetic surgery. For instance, in July 2021, Tianjin Medical University Cancer Institute and Hospital, research center and hospital based in China initiated a clinical trial study for evaluation of safety and effeciacy of TiLOOP Bra mesh in patients with expander-implant breast reconstruction. The researchers contend that combining TiLOOP Bra mesh with tissue expanders will decrease capsular contraction rates, improve the efficacy of expansion, and produce more aesthetically pleasing results of the cosmetic surgery. The study is estimated to get completed by the November 2023.
Asia Pacific Breast Implants Market : Key Developments
In October 2022, Second Affiliated Hospital, School of Medicine, Zhejiang University, a hospital based in China, in collaboration with Affiliated Hangzhou First People's Hospital, Zhejiang University, hospital based in China initiated the clinical trial study titled “Axillary Versus Primary Breast Approach for Second-stage Operation in Expander-Implant Breast Reconstruction”. The study is estimated to get completed by the December 2024.
In August 2022, BellaSeno GmbH, a medical technology manufacturing company announced that its proprietary 3D printed breast implants has entered human trials. BellaSeno’s 3D printed breast scaffolds are fully-resorbable, in that they are designed to be implanted during breast regeneration, augmentation or revision surgeries, before being slowly absorbed by the body.
In April 2022, GC Aesthetics (GCA), medical technology company, announced the it has gained the approval from the U.S. Food and Drug Administration for its new breast implant named LUNA xt micro-textured anatomical breast implant.
In May 2022, Prayasta 3D Inventions Pvt Ltd, Pune-based startup, won the national technology award for 2022 presented by the Department of Science and Technology (DST). It is an avid supplier of rupture-free 3D breast implants and prosthetics, by using its indigenously developed iEAM technology (elastomer additive manufacturing).
Asia Pacific Breast Implants Market : Restraint
Product Recall by the regulatory authority
The government is taking action to reduce the adverse events associated with the breast implants and breast augmentation surgeries. For instance, in July 2019, according to the data published by the National Center for Biotechnology Information, the U.S. Food and Drug Administration, requested Allergan, a pharmaceutical company to recall its Biocell textured breast implants because they had been linked to Breast Implant Associated Lymphoma (BIA-ALCL), or breast implant-associated anaplastic large cell lymphoma, a rare cancer. The U.S. Food and Drug Administration recommends women with BIA-ALCL to have their implants removed. Furthermore, in August 2022, the U.S. Food and Drug Administration reported that it had received 1,130 reports of BIA-ALCL, and 953 of those reports were related to Allergan implants. Moreover, in September 2022, the U.S. Food and Drug Administration received additional reports of other types of cancer not related to BIA-ALCL found in scar tissue of smooth and textured implants. Thus, product recall by the regulatory authority is the major factor that can hamper the growth of the Asia Pacific breat implants market over the forecast period. The launch of breast implants made with the bioresorbable polymer can reduce the adverse events of Breast Implant Associated Lymphoma (BIA-ALCL).
Asia Pacific Breast Implants Market : Key Players
Major players operating in the Asia Pacific breat implants market include Allergan, Plc. (AbbVie Inc.), Mentor Worldwide LLC, GC Aesthetics Plc, Sientra, Inc., POLYTECH Health and Aesthetics GmBH, Establishment Labs S.A., Laboratories Arion, HanSBiomed Co. Ltd., Groupe Sebbin SAS, and GG Biotechnology Ltd.
Definition: A breast implant is a prosthesis used to change the size, shape, and contour of a person's breast. In reconstructive plastic surgery, breast implants can be placed to restore a natural looking breast.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients