Bio-engineered Stent Market is estimated to be valued at USD 5,747.60 Mn in 2026 and is expected to reach USD 9,960.3 Mn in 2033, exhibiting a compound annual growth rate (CAGR) of 8.7% from 2026 to 2033.
Bio-engineered stent plays a major role in modern healthcare as it expands the blood vessel to prevent a blockage of arteries in cardiovascular diseases such as coronary heart disease and ischemic heart disease. The stents are commonly used with antibody coating to attract Endothelial Progenitor Cells (EPCs), which aids in quick rebuilding of the artery’s inner lining (endothelium) to prevent clots, and restenosis. This technique offers a fastest healing alternative to standard drug-eluting or bare-metal stents.
|
Current Event |
Description and its impact |
|
Regulatory Evolution in Medical Device Approval Processes |
|
|
Technological Breakthroughs in Biomaterial Engineering |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
|
Year |
Acquirer |
Target |
Focus |
Impact |
|
2024 |
Abbott |
Cardiovascular startup (unnamed) |
Bio‑absorbable scaffolds |
Strengthened leadership in resorbable stents |
|
2025 |
Boston Scientific |
Elutia’s vascular business |
Drug‑eluting biomaterial implants |
Expanded bio‑engineered stent portfolio in North America |
|
2025 |
Biotronik |
Collaboration with Texray (non‑stent) |
Radiation protection |
Indirect, but shows diversification |
|
2026 (expected) |
Multiple medtech firms |
Smaller biotech innovators |
Smart stents with sensors |
Anticipated consolidation to capture monitoring technologies |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of application type, the coronary artery disease segment is expected to hold 57.4% share of the market in 2026, because it is the most common cardiovascular disease in the world. Demand is rising due to its prevalence, the population is aging, and people are living unhealthy lives. Stents restore blood flow, lower the risk of heart attacks, and improve patient outcomes. This makes CAD interventions a significant component of the bio-engineered stent market.
For instance, in February 2025, the investigational bioadaptor is aimed at surpassing conventional stents in the management of coronary artery disease. It adapts to the physiology of the vessel, which may lower the risk of restenosis and thrombosis. This is different from drug-eluting stents. This new product shows how the bio-engineered stent market is changing, leading to better patient outcomes and signaling that new technologies will compete with outdated ones.
In terms of product type, the drug-eluting stents segment is expected to lead the market with 68.2% share in 2026, due to their ability to lower the risk of restenosis. They release medicines to prevent arteries from re-narrowing, which keeps the vessels open for a long time. Physicians prefer DES as they are reliable and have been tested in many clinical trials. By 2026, they will be the gold standard in interventional cardiology.
For instance, in May 2025, Boston Scientific's new drug-eluting stents for peripheral artery disease show that the bio-engineered stent market is growing. These stents release medicine to prevent restenosis, which makes the vessels more open and helps the patient. They strengthen the dominance of drug-eluting stents by expanding their use beyond the heart. This drives demand and opens up new uses in 2026's cardiovascular treatment landscape.
In terms of mode of delivery, the balloon-expandable stents segments is projected to account for 60% share of the market in 2026, because they allow precise positioning during coronary interventions. Their controlled expansion makes sure they are placed correctly, which lowers the risk of problems. They are widely used in catheterization labs and are preferred for coronary artery procedures where accuracy is extremely crucial. This gives them an edge over self-expanding options in the 2026 market.
In terms of material type, the metal-based stents segment is projected to capture 70% of the share in 2026, because they are stronger, more flexible, and more compatible with the body. Cobalt-chromium and stainless steel are two materials that are strong and work well under arterial pressure. Even with improvements in polymer technology, metals continue to represent the best choice for safety and long-term results. They are still going to make up the majority of the market in 2026.
In terms of end user, the hospitals and cardiac centers segment is estimated to acquire 71% share of the market in 2026, because they have advanced catheterization labs, skilled cardiologists, and places to care for patients after surgery. Hospitals are the main places where complex stent procedures are done due to their need special infrastructure. Their thorough patient management makes sure they get the most stent implants in 2026.
For instance, in November 2025, The Supernova stent from India is an important advancement forward in stroke treatment, expanding access through cost‑effective innovation. Hospitals and cardiac centers use them the most as they have better infrastructure, more skilled specialists, and better post-operative care.
To learn more about this report, Download Free Sample
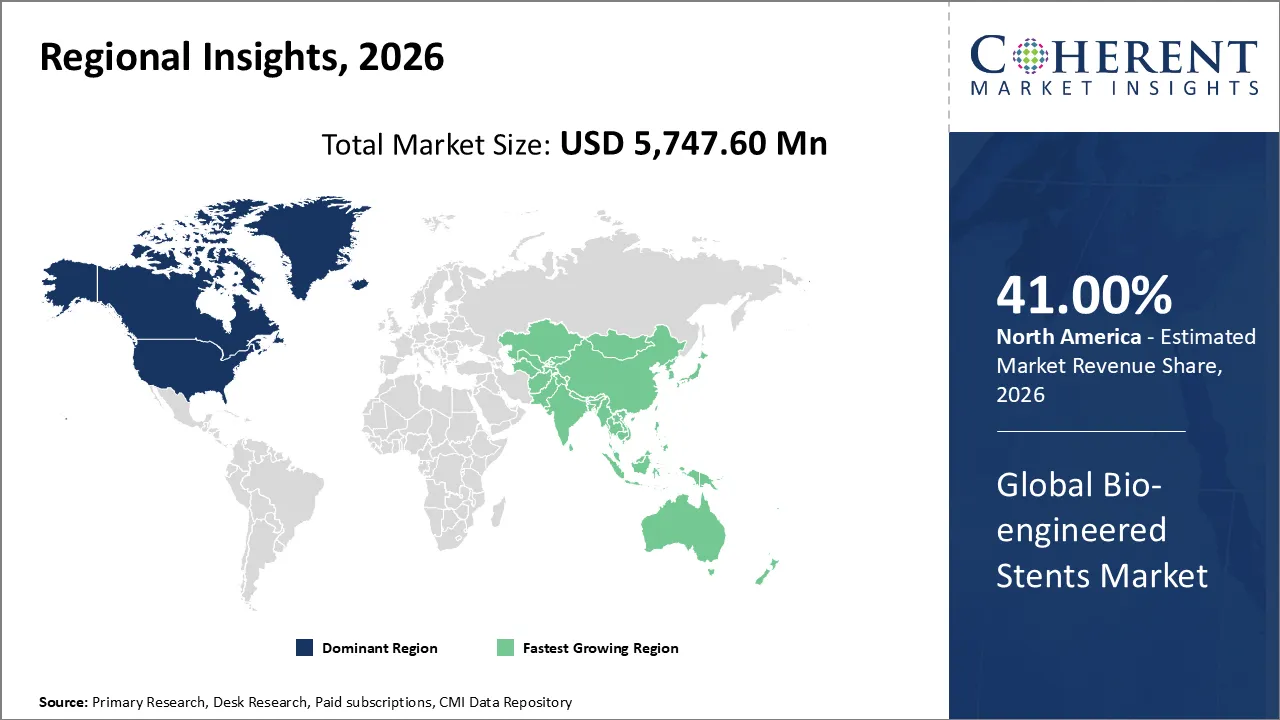
North America is expected to dominate the bio-engineered stent market with 41% share in 2026, due to coronary artery disease prevalence, healthcare infrastructure is getting better, and drug-eluting stents are becoming more popular. The region is the market leader given its favorable reimbursement policies, an aging population, and the fact that there are a lot of skilled cardiologists available.
For instance, in September 2025, Boston Scientific, which is based in Massachusetts, acquired Elutia's vascular business for $88 million. This made its cardiovascular portfolio stronger. The deal is mostly about bioengineered implants that release drugs, like stents, to help healing and lower the risk of complications.
Asia Pacific is expected to exhibited to the fastest growth, due to prevalence of cardiovascular disease, large aging population, and increasing unhealthy lifestyles. Adoption is driven by better healthcare infrastructure, government programs, and lower costs. Asia-Pacific is the fastest-growing region given its rapid urbanization and medical tourism.
For instance, in October 2025, Researchers at Hanyang University in South Korea developed ultrathin sensors integrated into stent grafts to fix aneurysms. These smart bio-engineered stents monitor on endoleaks all the time, which lowers the risk of rupture and makes patients safer. The innovation is originated from the Asia-Pacific region and makes the region's role in developing next-generation cardiovascular technologies in the global stent market even stronger.
The U.S. anticipates requiring more bio-engineered stents in 2026 because increasing prevalence of heart disease, the population will be getting older, and people will want more advanced drug-eluting and resorbable scaffolds. Strong hospital infrastructure, FDA approvals, and the use of smart monitoring stents are all factors that drive growth and make North America the leader in global stent innovation.
By 2026, the demand for bio-engineered stents in China are going to increase because rising prevalence heart disease, urban lifestyle risks, and the population is getting older. Adoption is driven by the growth of healthcare infrastructure, government investment, and the preference for advanced drug-eluting and resorbable scaffolds. China is a major driver of growth around the world because it has the most patients in the Asia-Pacific region.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 5,747.60 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.7% | 2033 Value Projection: | USD 9,960.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, plc., Boston Scientific Corporation, Abbott Laboratories, Biotronik SE & Co. KG, B. Braun Melsungen AG, Terumo Corporation, MicroPort Scientific Corporation, Stentys Sao, Meril Life Sciences Pvt. Ltd, Vascular Concepts, W. L. Gore and Associates, C. R. Bard, Endologix, Inc., Lombard Medical Technologies, Translumina GmbH, and JOTEC GmbH. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Coronary artery disease (CAD) and peripheral artery disease (PAD) are still two of the most common causes of death around the world., driven by aging population, living more sedentary lives, and rising diabetes and high blood pressure. Since these things, there is a steady demand for advanced stent technologies that help patients get better. As hospitals use new technologies, the bioengineered stent market share grows tremendously, reflecting growing reliance on drug-eluting and resorbable scaffolds to deal with the growing number of cardiovascular diseases around the world.
Drug eluting stents (DES) are the most popular choice right now as they release medicine and prevent restenosis. Resorbable scaffolds, on the other hand, provide temporary support for blood vessels and dissolve over time, restoring normal function. These devices are popular with hospitals as they have been shown to work well over time, which is why they are growing so quickly. This new technology is driving up demand for bioengineered stents since healthcare providers are putting funds into advanced stent technologies that offer safety, effectiveness, and benefits for patients. These technologies are becoming important drivers of the global cardiovascular device market.
Recent breakthroughs in biotechnology and material science are transforming cardiovascular care. Researchers are developing bio-engineered stents with enhanced cellular integration, promoting faster endothelialization and reducing restenosis risks. Innovations such as nanotechnology coatings and drug-eluting mechanisms improve patient outcomes by minimizing inflammation and thrombosis. These advancements not only extend stent lifespan but also open new opportunities for personalized medicine. According to the bio-engineered stent market forecast, such innovations will drive significant growth across global healthcare systems.
For instance, in April 2025, Researchers at ETH Zurich in Switzerland developed with ultrasound and artificial cilia technology to clean stents and catheters. This bioengineered new idea prevents biofilm from building up, which lowers the chances of infection and blockage. Europe is taking greater ownership in developing next-generation cardiovascular devices.
The clinical need for devices that improve vascular healing and lower long-term complications in comparison to conventional metallic implants is driving the global bio-engineered stent market in interventional cardiology. Due to better results in endothelial recovery and restenosis prevention, bio-engineered stents, which include drug-eluting, biodegradable, and bioabsorbable scaffold technologies are being used more frequently. Next-generation metal alloys and polymer-based coatings are examples of advanced material platforms that are essential to clinical differentiation and product innovation.
Additionally, to their proven performance and ease of handling, metal-based bio-engineered stents currently dominate clinical use. In contrast, polymer-based designs are advancing quickly owing to improved biocompatibility and regulated drug delivery profiles. With integrated catheterization infrastructure and procedural volumes that support them, hospitals and specialized cardiac centers continue to be the main users.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients