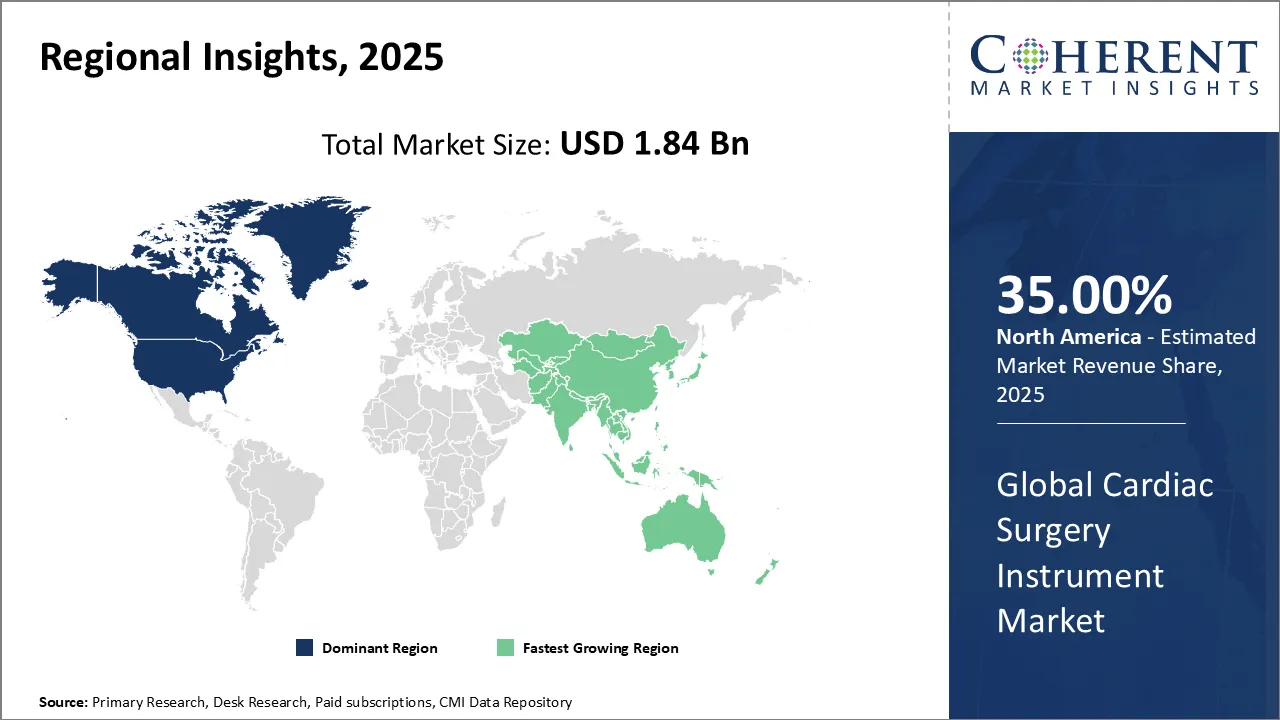
Cardiac Surgery Instrument Market is estimated to be valued at USD 1.84 Bn in 2025 and is expected to reach USD 2.41 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 3.9% from 2025 to 2032.
The Cardiac Surgery Instrument Market demand is witnessing steady growth, driven by the rising prevalence of cardiovascular diseases, increasing geriatric population, and advancements in minimally invasive cardiac procedures. Surgeons are adopting innovative instruments for enhanced precision, safety, and efficiency. Growing hospital infrastructure and rising awareness about cardiac health are further fueling Cardiac Surgery Instrument Market demand globally.
|
Current Event |
Description and its Impact |
|
Regulatory Evolution and Compliance Challenges |
|
|
Demographic and Disease Burden Transformation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
AI plays a transformative role in cardiac surgery instruments by enhancing precision, safety, and efficiency. Intelligent systems assist surgeons through real-time data analysis, improved imaging, and robotic guidance, enabling minimally invasive procedures with greater accuracy. AI-driven tools can predict complications, optimize surgical plans, and monitor patient vitals during operations. Machine learning algorithms also help in instrument calibration and adaptive control, reducing human error and procedure time.
In August 2025, Narayana Health developed an AI model that detects heart failure from standard ECG images within 10 seconds. Created by the hospital’s Clinical Research Team and Medha AI, it predicts left ventricular ejection fraction (EF) to identify early heart failure signs quickly. Designed to aid over 10 million Indians with limited access to echocardiography, the model was trained on 100,000+ ECG and echo pairs and achieved 97% accuracy in detecting severely reduced EF (≤35%) in a multi-centre study of 57,000 patients.
In terms of application, the Coronary Artery Bypass Grafting (CABG) segment is projected to account for 43% share in 2025, driven by the high prevalence of coronary artery disease and increasing surgeries globally. Rising adoption of minimally invasive and robotic-assisted techniques, coupled with advancements in surgical instruments for precision and safety, boosts Cardiac Surgery Instrument Market demand, particularly in North America and Europe.
For instance, in September 2025, Aster Medcity Hospital in Kochi performed a robotic-assisted coronary artery bypass grafting (CABG) on a 42-year-old woman with severe triple vessel disease. Opting for a minimally invasive direct coronary artery bypass (MIDCAB) procedure, the patient was discharged just four days post-surgery. The advanced robotic system enabled enhanced precision and faster recovery, with reduced scarring and complications.
In terms of product type, the catheters & guidewires segment is expected to hold the largest share of the market in 2025. This dominance is driven by the increasing prevalence of cardiovascular diseases, advancements in minimally invasive procedures, and the growing demand for diagnostic and therapeutic interventions. Catheters and guidewires play a crucial role in procedures such as angioplasty, stent placement, and electrophysiological studies, contributing significantly to the market's growth. Additionally, the rise in outpatient procedures and the shift towards less invasive treatments further bolster the demand for these instruments.
For instance, in October 2024, Boston Scientific introduced Journey™ Guidewire, a 0.014" guidewire designed for challenging, small-vessel peripheral angioplasty procedures. The device enhances precision and tracking, addressing physician needs in treating arteries below the knee. Dr. James Benenati, Medical Director at Baptist Cardiac and Vascular Institute, praised its exceptional torque and flexibility in complex cases.
In terms of end user, the hospital segment is expected to capture the highest share of the market in 2025, due to factors such as the increasing prevalence of cardiovascular diseases, advancements in surgical technologies, and the growing number of cardiac procedures performed in hospital settings. Hospitals offer comprehensive facilities, specialized medical staff, and advanced equipment, making them the primary end-users of cardiac surgery instruments. While Ambulatory Surgical Centers (ASCs) and Research Institutes also contribute to the market, their share is comparatively smaller due to limited infrastructure and specialized services. Therefore, Hospitals are expected to maintain the largest market share in the cardiac surgery instrument sector in 2025.
For instance, in June 2025, Cardinal Health launched the Kendall DL™ Multi System, a single-use monitoring device that continuously tracks cardiac activity, blood oxygen levels, and body temperature through a single connection. Designed for patient use from admission to discharge, it enhances clinician workflows and reduces cross-contamination risks associated with reusable lead wires. The system also minimizes false alarms and motion artifacts in ECG tracings, improving clinical efficiency.
To learn more about this report, Download Free Sample
North America is the largest market for cardiac surgery instrument, accounting for a share of over 35.0% in 2025 North America has historically been a major market for cardiac surgery instruments due to the presence of advanced healthcare infrastructure, skilled medical professionals, and a high prevalence of cardiovascular diseases. The U.S. and Canada are key contributors to the market. Technological innovations and a focus on minimally invasive procedures have driven market growth in this region.
For instance, in May 2025, Johnson & Johnson MedTech introduced the SOUNDSTAR CRYSTAL™ Ultrasound Catheter in the U.S., enhancing 2D intracardiac imaging during cardiac ablation procedures. This advanced catheter offers superior image clarity and integrates seamlessly with the CARTO™ 3 Mapping System, including the AI-powered CARTOSOUND™ FAM module, improving workflow efficiency for electrophysiologists.
Asia Pacific is the fastest-growing market for cardiac surgery instrument, accounting for a share of over 29.5% in 2025. The Asia-Pacific region has shown rapid growth in the healthcare sector, including cardiac surgery. Countries like China, India, Japan, and South Korea have witnessed an increase in healthcare spending, leading to improved healthcare facilities and greater access to advanced medical equipment. Rising cardiovascular disease rates and an aging population have also contributed to the demand for cardiac surgery instruments.
For instance, in July 2025, Johnson & Johnson MedTech launched its VARIPULSE™ Platform across the Asia-Pacific region to enhance atrial fibrillation (AFib) treatment. This platform integrates pulsed field ablation (PFA) technology with the CARTO™ 3 electroanatomical mapping system, enabling precise, real-time visualization during catheter ablation procedures. Approved in Japan, Hong Kong, China, Australia, Taiwan, and Korea, the VARIPULSE™ Platform aims to improve procedural accuracy and patient outcomes in AFib care.
The United States dominates the global Cardiac Surgery Instrument Market due to a high number of cardiac procedures, well-established healthcare infrastructure, and significant investments in advanced medical technologies. Strong adoption of minimally invasive surgeries, presence of leading hospitals, and continuous innovation in surgical instruments further drive market growth, ensuring high demand for precise and reliable cardiac surgery tools.
For instance, in August 2025, Cleveland Clinic performed the world's first robotic-assisted aortic valve replacement using Corcym's Perceval Plus sutureless valve. The minimally invasive procedure, known as AVATAR (Advanced Videoscopic Aortic valve surgery by Transcervical Approach using Robot assistance), was conducted through a small incision in the patient's neck. This innovative approach, combining Corcym's valve with CardioPrecision's CoreVista Robot Enabling Platform, aims to reduce patient recovery time and hospital stay.
Japan’s demand for cardiac surgery instruments is fueled by its rapidly aging population, which increases the prevalence of cardiovascular diseases requiring surgical interventions. Advanced healthcare infrastructure, high adoption of cutting-edge medical technologies, and government support for modern medical procedures further drive the market. Hospitals and specialized cardiac centers prioritize state-of-the-art instruments to improve surgical outcomes and patient care.
For instance, in September 2025, Toray Industries introduced the "Inoue Balloon™ for Aortic Valve," a balloon valvuloplasty catheter designed to treat aortic valve stenosis. This launch follows the receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA), marking a significant advancement in cardiovascular medical devices.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.84 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 3.9% | 2032 Value Projection: | USD 2.41 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Murray Surgical, SternMed GmbH, CARDIC INSTRUMENTS, Edwards Lifesciences Corporation., Abbott, Boston Scientific Corporation, LivaNova, Inc., Terumo Corporation, Getinge, Artivion, Inc., MicroPort Scientific Corporation, B. Braun SE, and Stryker |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The cardiac surgery instruments market value is undergoing a significant transformation, driven by technological advancements and evolving surgical practices. A notable trend is the increasing adoption of robotic-assisted surgeries, which are enhancing precision and reducing recovery times. For instance, Intuitive Surgical's da Vinci system has revolutionized minimally invasive procedures by offering enhanced dexterity and control. The introduction of the da Vinci 5 model further refines these capabilities, promising improved patient outcomes and operational efficiency.
In parallel, artificial intelligence (AI) is making inroads into cardiac surgery. A recent development in Chile showcased an AI-guided autonomous camera system that enabled a surgeon to perform a gallbladder removal surgery solo. This innovation exemplifies the potential of AI to support surgical procedures, offering real-time adjustments and enhancing procedural accuracy.
Geographically, North America continues to lead the market, accounting for a substantial share due to its advanced healthcare infrastructure and high healthcare spending. However, the Asia-Pacific region is emerging as a significant growth area, driven by increasing healthcare expenditures and improved access to advanced surgical instruments.
*Definition: Cardiac surgery instruments are specialized medical devices and tools designed for use during surgical procedures related to the heart and cardiovascular system. These instruments assist surgeons in performing various cardiac interventions, including coronary artery bypass grafting, heart valve repair/replacement, and other procedures aimed at treating heart conditions.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients