Global cell therapy manufacturing market is estimated to be valued at USD 5.65 Bn in 2025 and is expected to reach USD 15.14 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 15.1% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
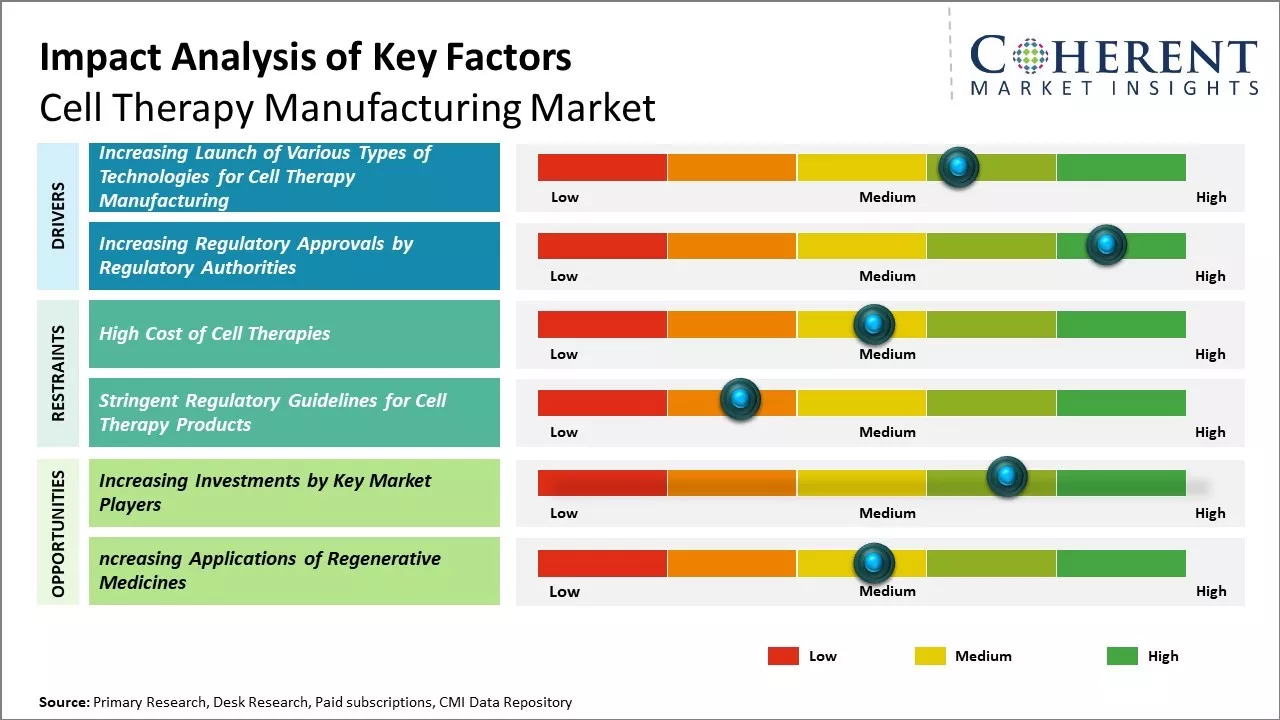
Increasing prevalence of chronic diseases such as cancer and cardiovascular diseases boosts demand for cell therapy products across the world. The advancements in cell therapy research and development and rise in the number of clinical trials are driving the market growth. Various market players are constantly focused on developing innovative cell therapy products, in order to expand their market share. Regulatory complexity and high costs involved can hamper the growth of the cell therapy manufacturing market.
Increasing Launch of Various Types of Technologies for Cell Therapy Manufacturing
Manufacturers are involved in developing and launching various types of technologies for cell therapy manufacturing, and this is expected to drive the growth of global cell therapy manufacturing market. For instance, in July 2022, INVETECH, a global company offering solutions, systems, and services to cell and gene therapy, announced the launch of new korus technology for autologous cell therapy production. Korus is a closed system for the production of autologous cell therapy.
Get actionable strategies to beat competition: Download Free Sample
Increasing Regulatory Approvals by Regulatory AuthoritiesManufacturers of cell therapy product are focusing on receiving regulatory approvals to enhance their cell therapy manufacturing process, and this is expected to drive the global cell therapy manufacturing market growth. For instance, in May 2022, Bristol Myers Squibb Company, a pharmaceutical company, announced that the U.S. Food and Drug Administration had approved Breyanzi (lisocabtagene maraleucel; liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).

To learn more about this report, Download Free Sample
Market Challenge – High Cost of Cell TherapiesHigh cost of cell therapies is one of the biggest challenges, with financial implications for patients, payers, and providers, and this is expected to hamper the market growth over the forecast period. For instance, in May 2022, chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (Kymriah) received the U.S. FDA approval for the treatment of pediatric and young adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).It costs around US$ 475,000 for a single infusion. This has resulted in limited adoption of such therapies, thus, hampering growth of the market. Moreover, in April 2022, Yescarta, a cell therapy, developed by Kite Pharma, Inc., a pharmaceutical company, was approved by the U.S. FDA for the treatment of certain B-cell lymphomas. The listed price for axicabtagene ciloleucel (Yescarta) in the U.S. is US$ 373,000.
Market Opportunity– Increasing Investments by Key Market Players
Increasing adoption of inorganic growth strategies such as investments is expected to drive growth of the global cell therapy manufacturing market. For instance, on February 6, 2024, AstraZeneca, a global pharmaceutical company, announced that it had invested US$ 300 million in a facility in Rockville, Maryland, U.S. for discovery and development of cell therapies. The aim of the investment is to launch its life-saving cell therapy platforms in the U.S. for critical cancer trials and future commercial supply.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
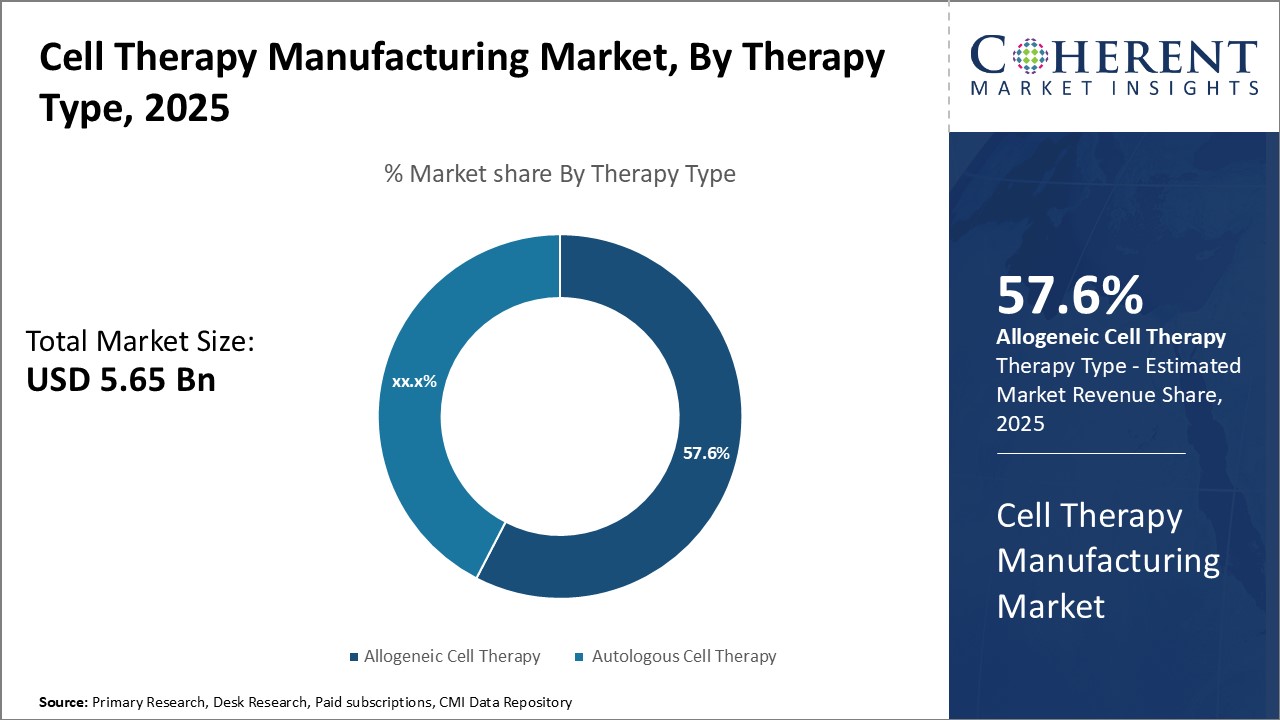
Insights, By Therapy Type: Immunological compatibility drives the growth of allogeneic cell therapy segmentTherapy Type segment is sub-segmented into allogeneic cell therapy and autologous cell therapy. Allogeneic cell therapy sub-segment is estimated to hold 57.6% of the market share in 2025 due to its immunological compatibility advantages over autologous therapies. Allogeneic cell therapy involves the use of donor cells that are not genetically identical to the recipient. With allogeneic cell therapies, large batches of unmodified donor cells can be mass produced in advance and stored for immediate off-the-shelf use by multiple patients. This efficient production process and immediacy of treatment provides significant advantages over customized autologous therapies.
Insights, By Manufacturing Purpose: Regenerative medicine drives growth of clinical manufacturing uses
Manufacturing Purpose segment is sub-segmented into clinical, commercial, pre-clinical. The clinical sub-segment is estimated to hold 64% of the market share in 2025 due to the expanding applications of these therapies for regenerative medicine. The clinical manufacturing of cell therapies primarily serves research and trials focused on exploring the regenerative potential of cell treatments for various diseases. A major factor driving the clinical manufacturing segment is the growing body of evidence demonstrating the ability of cell therapies to reverse tissue damage, regenerate lost cells and structures, relieve symptoms, and potentially cure certain conditions. Promising clinical results have been shown for cell therapies applied to regenerate tissues affected by cardiovascular disease, neurological disorders, musculoskeletal injuries, and other degenerative conditions. The ability of cells like mesenchymal stem cells to differentiate into tissues like bone, cartilage, heart muscle and nerve cells underlies their therapeutic regenerative functions. Optimism around the continued progress and commercialization of new regenerative applications is sustaining strong investment in the clinical manufacturing of cell therapy products.
Insights, By Application: Increasing launch of joint venture for oncology
Application segment is sub-segmented into musculoskeletal, cardiovascular, gastrointestinal, neurological, oncology, dermatology, others. Oncology sub-segment is estimated to hold 33.6% of the market share in 2025 due to increasing launch of joint venture for oncology. For instance, in June 2022, the University of Texas MD Anderson Cancer Center and National Resilience, Inc., a biotechnology company, announced the launch of a joint venture, the Cell Therapy Manufacturing Center (CTMC), to accelerate the development and manufacturing of innovative cell therapies for patients with cancer.
Need a Different Region or Segment? Download Free Sample
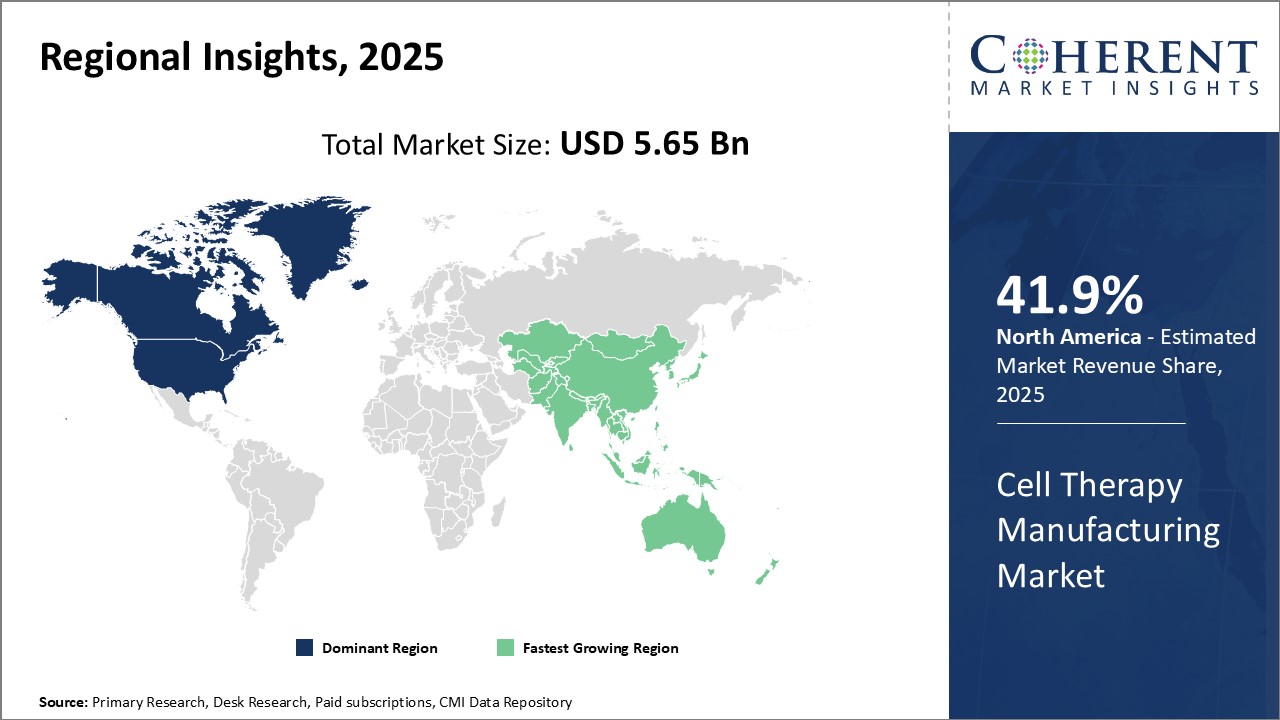
North America remains the dominant region in the global cell therapy manufacturing market and is estimated to hold 41.9% of the market share in 2025. The presence of leading biopharmaceutical companies and research institutions focused on cell therapies has fueled extensive research and development activities in the region. Countries like the U.S. have supportive regulations and reimbursement policies spurring clinical trials and commercialization of new cell therapy products. Access to capital for funding clinical trials along with increasing healthcare expenditure provides North American players an advantage. Several major players have set up their manufacturing facilities in the U.S. to cater to the large patient pool and tap into the growing demand. Over the years, these have built capabilities across the manufacturing value chain from cell sourcing and processing to final drug product formulation, storage, and distribution.
Asia Pacific has emerged as the fastest-growing regional market for cell therapy manufacturing. Countries like China and Japan recognize the potential of cell-based therapies, and are aggressively supporting industry growth. Their governments provide funding and infrastructure development initiatives to develop domestic capabilities. Lower operating costs and talent availability in the regions are attracting investments from global industry players seeking to expand production footprints. Local companies in Asia have ramped up technology innovations centered around process intensification. The rapid economic growth across Asia Pacific translates to increased healthcare spending and demand for advanced treatment options like cell therapies. This makes the region an attractive long-term investment destination for both regional as well as global manufacturers.
Cell Therapy Manufacturing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 5.65 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15.1% | 2032 Value Projection: | USD 15.14 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
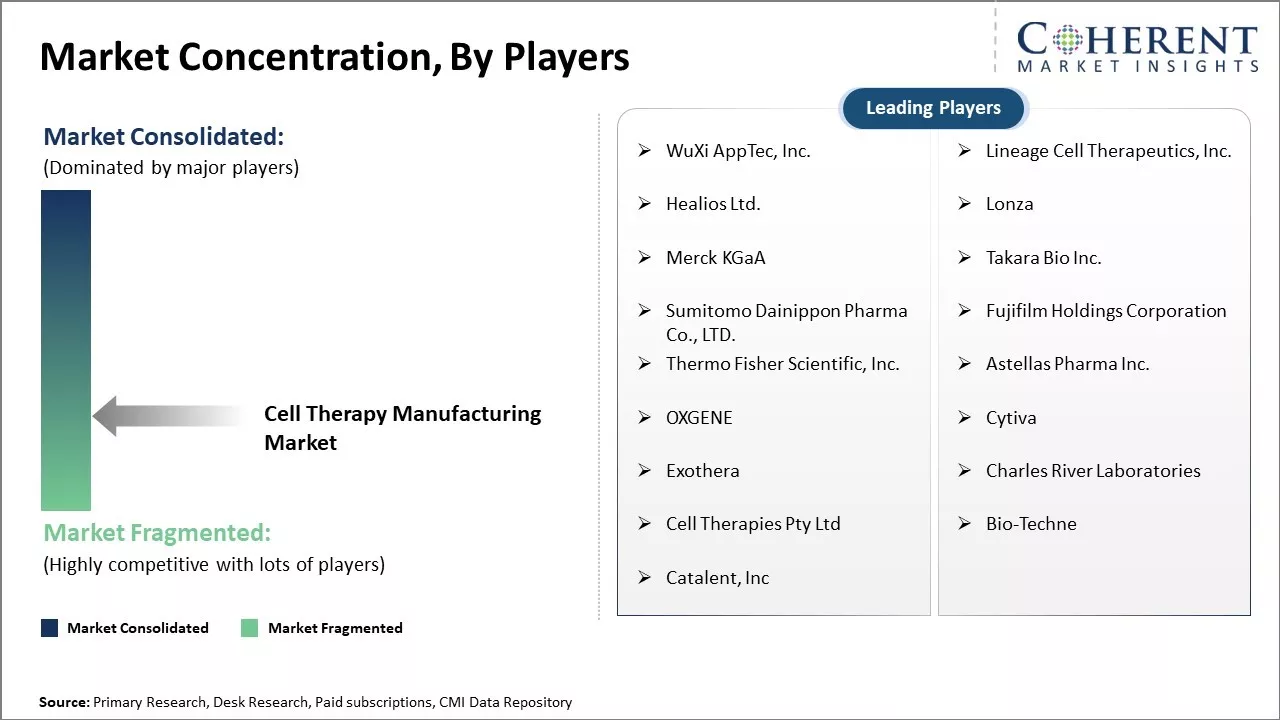
WuXi AppTec, Inc., Lineage Cell Therapeutics, Inc., Healios Ltd., Lonza , Merck KGaA, Takara Bio Inc., Sumitomo Dainippon Pharma Co., LTD., Fujifilm Holdings Corporation, Thermo Fisher Scientific, Inc., Astellas Pharma Inc., OXGENE, Cytiva, Exothera, Charles River Laboratories, Cell Therapies Pty Ltd, Bio-Techne, Catalent, Inc |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Cell therapy is the transplantation of human cells to replace or repair damaged tissue or cells. Numerous different types of cells may be used as part of a therapy or treatment for a number of diseases and disorders with the help of new technologies, inventive products, and infinite imagination. Hematopoietic (blood-forming) stem cells (HSC), skeletal muscle stem cells, mesenchymal stem cells, lymphocytes, dendritic cells, and pancreatic islet cells are a few of the cells that could be used.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients