Circulating Cell-Free Tumor DNA Market is estimated to be valued at USD 9.22 Bn in 2025 and is expected to reach USD 39.05 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 22.9% from 2025 to 2032.
Analysts’ Views on Global Circulating Cell-free Tumor DNA Market:
The global Circulating Cell-free Tumor DNA Market's growth can be hindered by disadvantages associated with circulating cell-free tumor DNA analysis like some tests require prior knowledge of tumor mutations, results can be semiquantitative,etc. The use of circulating cell-free tumor DNA tests for the diagnosis of cancer is expected to drive the global Circulating Cell-free Tumor DNA Market over the forecast period. For instance, in January 2022, a report published by Springer Nature Limited, a scientific journal, stated that cell-free tumor DNA analysis represents a promising method for the diagnosis, treatment selection, and clinical follow-up of cancer patients.
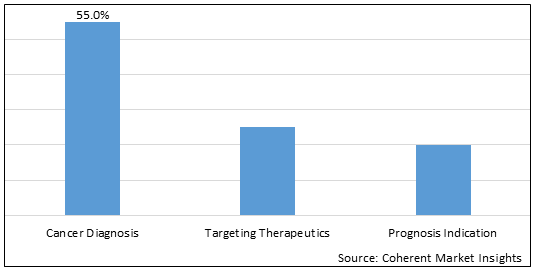
Figure 1. Global Circulating Cell-free Tumor DNA Market Share (%), by Application, 2025
To learn more about this report, Download Free Sample
Global Circulating Cell-free Tumor DNA Market – Driver
Increasing product launches by key players in the market
Increasing product launches by key players in the market to expand their product portfolio is expected to drive market growth over the forecast period. For instance, Foundation Medicine, a manufacturer of genomic profiling assays based on next-generation sequencing technology, and Natera, a global leader in cell-free DNA (cfDNA) testing announced the launch of an early access program for the investigational and clinical use of FoundationOne Tracker, a personalized circulating tumor DNA (ctDNA) monitoring assay.
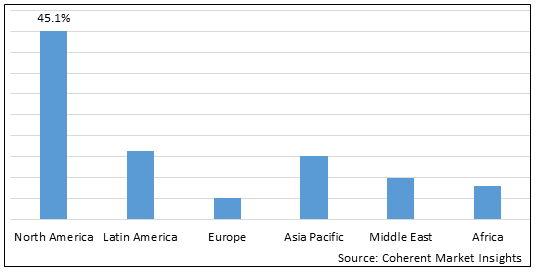
Figure 2. Global Circulating Cell-free Tumor DNA Market Value (US$ billion), by Region, 2025
To learn more about this report, Download Free Sample
Global Circulating Cell-free Tumor DNA Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global Circulating Cell-free Tumor DNA Market over the forecast period. North America holds 45.1% of the market share due to the increasing prevalence of cancer in the region. For instance, according to the American Cancer Society, a scientific cancer journal, in the year 2021, an estimated 1.9 million new cancer cases were diagnosed and 608,570 cancer deaths occurred in the U.S. due to cancer.
Global Circulating Cell-free Tumor DNA Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
The COVID-19 pandemic had a negative impact on the global Circulating Cell-free Tumor DNA Market. For instance, in September 2020, according to an article published by the American Society of Clinical Oncology, a professional organization committed to conquering cancer through research, education, prevention, and delivery of high-quality patient care, stated that a detrimental impact of the COVID-19 pandemic on cancer care was observed during the COVID-19 pandemic.
Circulating Cell-free Tumor DNA Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 9.22 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 22.9% | 2032 Value Projection: | USD 39.05 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Biocept, Inc., Illumina, Inc., Quest Diagnostics Incorporated, KURABO INDUSTRIES LTD, PerkinElmer chemagen Technologie GmbH, Biodesix, Guardant Health, QIAGEN, Sequenom, Inc., Agilent Technologies, Inc., Fluxion Biosciences Inc., Natera, Inc., Agena Bioscience, Inc., Paragon Genomics, Inc., Lucence Health Inc., Eurofins Genomics, Thermo Fisher Scientific. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Circulating Cell-free Tumor DNA Market Segmentation:
The global Circulating Cell-free Tumor DNA Market report is segmented into Tumor Type, Cancer Type, Technology, Application, and Region
Based on Tumor Type, the market is segmented into malignant tumors and precancerous tumors. Out of which, the precancerous tumors segment is expected to hold a dominant position in the Circulating Cell-free Tumor DNA Market during the forecast period and this is attributed to the increasing usage of circulating cell-free tumor DNA analysis for early detection of cancer.
Based on Cancer Type, the market is segmented into lung cancer, colorectal cancer, breast cancer, and others. Out of which, the lung cancer segment is expected to hold a dominant position in the Circulating Cell-free Tumor DNA Market during the forecast period and this is attributed to the increasing prevalence of lung cancer.
Based on Technology, the Circulating Cell-free Tumor DNA Market is segmented into PCR, massively parallel sequencing, and single nucleotide polymorphism. The massively parallel sequencing segment is expected to dominate the market over the forecast period and this is to the increasing usage of massively parallel sequencing for the detection of cancer DNA.
Based on Application, the Circulating Cell-free Tumor DNA Market is segmented into cancer diagnosis, targeting therapeutics, and prognosis indication. The cancer diagnosis segment is expected to dominate the market over the forecast period and this is to the increasing usage of cancer diagnosis methods.
Among all segmentation, the application segment has the highest potential due to the increasing prevalence of cancer over the forecast period. For instance, in April 2021, according to a report published by American Medical Association, a scientific community, stated that Lung cancer is estimated to continue as the leading cause of cancer-related death in 2040 with an estimated 63,000 deaths.
Global Circulating Cell-free Tumor DNA Market Cross Sectional Analysis:
In cancer type segment, lung cancer holds a dominant segment in North America region due to the increasing prevalence of lung cancer. For instance, in November 2022, an article published by Jobson Medical Information LLC, a premier healthcare information and marketing services company, stated that approximately 236,740 new cases of lung cancer were projected in the year 2022.
Global Circulating Cell-free Tumor DNA Market: Key Developments
Global Circulating Cell-free Tumor DNA Market: Key Trends
Increasing prevalence and factors associated with cancer
Increasing prevalence of cancer needs effective factors for the management of cancer which is expected to drive the monoclonal antibody therapeutics market growth over the forecast period. For instance, in February 2021, an article published by the American Cancer Society, a scientific journal, stated that almost 10.0 million cancer deaths occurred in the year 2020.
Global Circulating Cell-free Tumor DNA Market: Restraint
Disadvantages of circulating cell-free tumor DNA analysis
The global Circulating Cell-free Tumor DNA Market can be hindered by disadvantages associated with circulating cell-free tumor DNA. For instance, in January 2022, a report published on MDPI, a publisher of open-access scientific journals, stated the disadvantages of cell-free tumor DNA detection are that some tests require prior knowledge of tumor mutations, results can be semi-quantitative, the source of cfDNA can be from multiple sources (apoptosis, graft rejection, immune cells vs. tumor cells, etc.). ) and analytical methods may involve technical difficulties.
Global Circulating Cell-free Tumor DNA Market - Key Players
Major players operating in the global Circulating Cell-free Tumor DNA Market include Biocept, Inc., Illumina, Inc., Quest Diagnostics Incorporated, KURABO INDUSTRIES LTD, PerkinElmer chemagen Technologie GmbH, Biodesix, Guardant Health, QIAGEN, Sequenom, Inc., Agilent Technologies, Inc., Fluxion Biosciences Inc., Natera, Inc., Agena Bioscience, Inc., Paragon Genomics, Inc., Lucence Health Inc., Eurofins Genomics, Thermo Fisher Scientific.
Global Circulating Cell-free Tumor DNA Market– Insights
The term "circulating tumor DNA" (ctDNA) refers to DNA from malignant cells and tumors that circulate in the bloodstream. The nucleus of a cell contains most of the DNA. Cells die and are replaced by new ones as a tumor expands. The broken-down dead cells discharge their contents, including DNA, into the bloodstream. ctDNA are short segments of DNA that are typically less than 200 nucleotides in length.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients