Elisa Analyzer Market Size and Forecast – 2025 – 2032
The Global Elisa Analyzer Market size is estimated to be valued at USD 1.45 billion in 2025 and is expected to reach USD 2.68 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Global Elisa Analyzer Market Overview
ELISA analyzers are laboratory instruments used to automate and enhance enzyme-linked immunosorbent assay (ELISA) procedures, a key diagnostic tool for detecting antigens, antibodies, and proteins in biological samples. These analyzers range from semi-automated systems for small laboratories to fully automated, high-throughput platforms used in hospitals and research institutions.
Key features include microplate reading, liquid handling, and automated data processing, which improve accuracy and reduce manual errors. Advanced models integrate software for data management and connectivity with laboratory information systems (LIS). Applications span infectious disease diagnosis, cancer biomarker testing, hormone analysis, and drug discovery, with growing demand from clinical diagnostics, biotechnology, and pharmaceutical sectors.
Key Takeaways
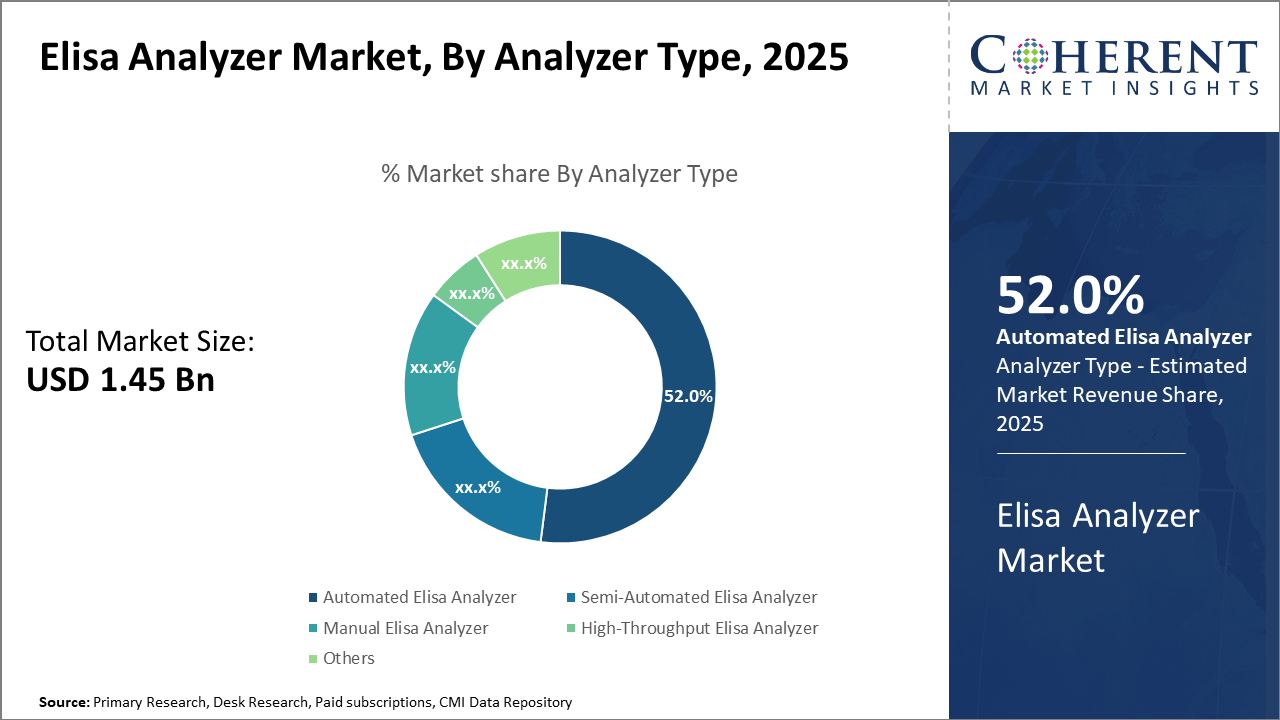
Automated ELISA analyzers dominate the analyzer type segment, accounting for 52% of market share, driven by efficiency demands in clinical diagnostics. Meanwhile, high-throughput analyzers are the fastest-growing subsegment due to increasing pharmaceutical R&D demands.
In terms of application, clinical diagnostics remains the largest segment, fueled by growing incidences of chronic diseases and infection management, whereas pharmaceutical industry applications are witnessing rapid expansion due to innovation in drug discovery processes.
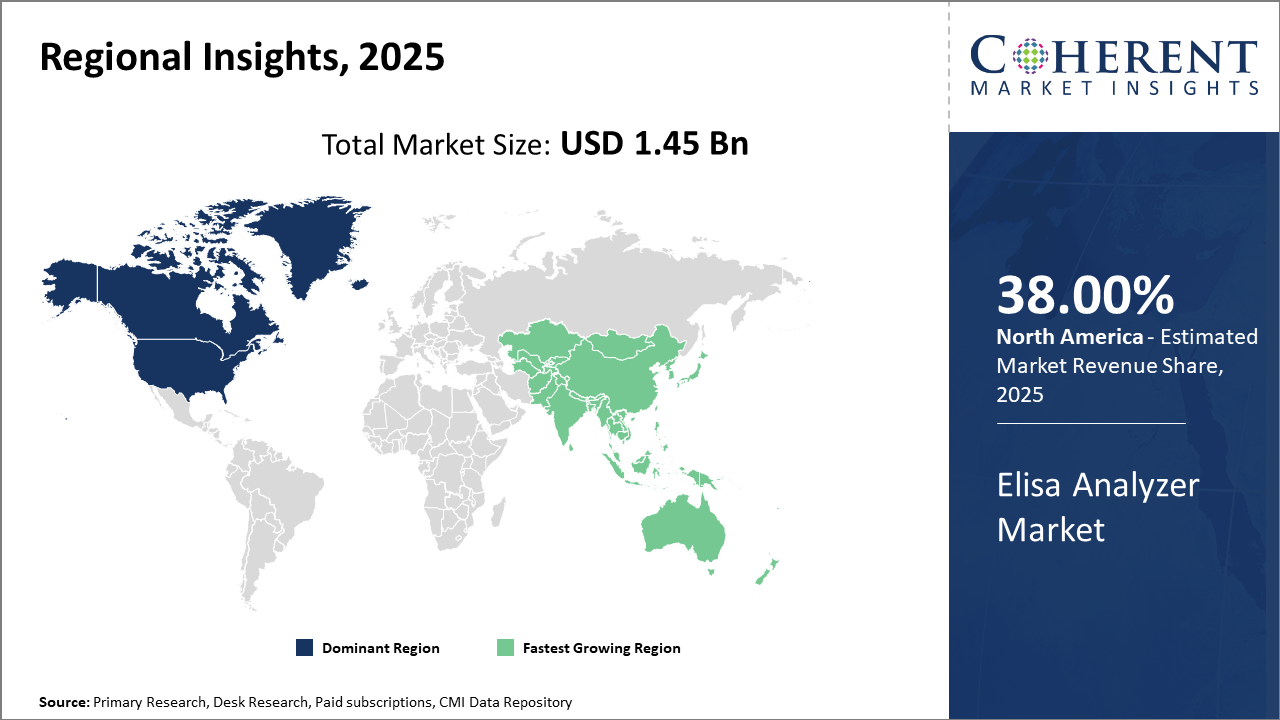
North America leads in market share with over 38% of the Elisa Analyzer industry value, driven by sophisticated healthcare infrastructure and high R&D investments.
Asia Pacific is the fastest-growing region, exhibiting a CAGR exceeding 11%, supported by rising investments in healthcare diagnostics and expanding government healthcare subsidies.
Elisa Analyzer Market Segmentation Analysis
To learn more about this report, Download Free Sample
Elisa Analyzer Market Insights, By Analyzer Type
Automated ELISA Analyzer dominates the market share. They are favored for their high reliability, precision, and reduced human error, making them the backbone for clinical and pharmaceutical testing applications globally. The segment’s growth is propelled by technological advancements like robotics-enabled processing and software integration for data management. Meanwhile, the High-Throughput ELISA Analyzer is the fastest-growing subsegment, driven by the pharmaceutical industry’s escalating demand for screening large sample volumes, especially in biomarker discovery and vaccine development.
Elisa Analyzer Market Insights, By Application
Clinical Diagnostics dominate the application segment, representing the highest industry share due to its critical role in disease detection and patient monitoring, supported by rising chronic illnesses and infectious disease prevalence globally. The Pharmaceutical Industry application is the fastest-growing subsegment, leveraging ELISA technology for drug discovery, pharmacokinetics, and clinical trials, with increasing R&D investments fueling demand. Food and Environmental Testing applications address regulatory compliance and safety testing, maintaining steady market revenue, while Research Laboratories utilize ELISA analyzers for proteomics and molecular biology research tailored to specialized demands.
Elisa Analyzer Market Insights, By End-User
Diagnostic Centers hold the largest market share due to extensive adoption for routine clinical testing and infectious disease management, underscored by continual growth in healthcare demand globally. CROs and Pharmaceutical Companies represent the fastest-growing subsegments, as outsourcing and in-house drug development increasingly depend on high-throughput ELISA Analyzers for biomarker analysis and assay development. Research Institutes utilize ELISA analyzers primarily for experimental and exploratory research, while other users include food safety labs and environmental testing agencies with niche but stable demand.
Elisa Analyzer Market Trends
Recent market trends indicate a decisive shift towards incorporating digital technologies into Elisa Analyzers.
For example, Europe has witnessed a 30% surge in the adoption of automated ELISA platforms integrated with cloud-based data analytics between 2023 and 2025.
Simultaneously, Asia Pacific is advancing rapidly in the miniaturization of ELISA devices suitable for point-of-care testing, with over 20% annual growth reported in portable assay demand in 2024.
Another notable development is the increased emphasis on environmentally sustainable assay reagents and consumables, driven by regulatory pressure in North America since 2024.
Elisa Analyzer Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Elisa Analyzer Market Analysis and Trends
In North America, the dominance in the ELISA Analyzer market is rooted in a mature healthcare ecosystem equipped with cutting-edge infrastructure and high R&D expenditure. The U.S. accounts for the majority of market revenue with large-scale adoption in hospitals and research institutes. Strong regulatory bodies and well-funded public health programs also contribute to innovation and market dynamics.
Asia Pacific Elisa Analyzer Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR surpassing 11%. This is fueled by expanding healthcare access, governmental initiatives on diagnostic affordability, and the rising prevalence of infectious diseases. Robust investments by leading companies to establish manufacturing and distribution hubs in India and China also amplify this growth trajectory.
Elisa Analyzer Market Outlook for Key Countries
USA Elisa Analyzer Market Analysis and Trends
The USA’s Elisa Analyzer market remains the largest globally, underpinned by substantial healthcare spending and stringent diagnostic accuracy requirements. The presence of leading industry companies such as Thermo Fisher Scientific and Danaher with headquarters and manufacturing plants here enhances innovation and supply chain robustness. The country’s hospital networks and pharmaceutical R&D centers have demonstrated a 15% year-on-year increase in advanced ELISA analyzer procurement in 2024, reflecting robust market maturity and revenue generation.
China Elisa Analyzer Market Analysis and Trends
China’s market is expanding rapidly due to increasing government healthcare initiatives aimed at improving diagnostic infrastructure, particularly in Tier 2 and Tier 3 cities. The rising burden of chronic and infectious diseases drives demand for high-throughput and automated Elisa devices. Leading players, including Bio-Rad Laboratories and PerkinElmer, have intensified local partnerships and production facilities, enabling a 20% sales growth recorded in 2025. Regulatory reforms also facilitate quicker product approval,s contributing to dynamic market growth in China.
Analyst Opinion
Growing demand for high-throughput Elisa Analyzers in clinical diagnostics is a primary driver of the market size. For instance, in 2024, hospital adoption in the U.S. increased by over 18%, highlighting supply-side expansion driven by capacity enhancement initiatives in major medical institutions.
Pricing optimization strategies focused on mid-sized laboratories have stimulated market revenue. Data from recent years show that cost-effective Elisa Analyzer models accounted for nearly 40% of new sales in the Asia Pacific in 2025, reflecting rising accessibility for emerging markets.
Expansion of at-home and point-of-care testing applications utilizing compact Elisa Analyzers is accelerating market growth. A prominent example is the rising deployment of portable Elisa Analyzer systems by major healthcare networks in Europe, with a reported 22% increase in demand in 2024.
Rising R&D investments targeting multi-analyte detection capability significantly influence market dynamics. Leading laboratory research facilities in North America enhanced their usage of multiplex Elisa Analyzers by 25% in 2025, driven by growing demand in biomarker profiling and pharmaceutical development.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.45 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 2.68 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Thermo Fisher Scientific, Bio-Rad Laboratories, PerkinElmer, Inc., Agilent Technologies, Siemens Healthineers, Merck KGaA, Abbott Laboratories, Promega Corporation, Molecular Devices LLC, QIAGEN N.V., Becton Dickinson and Company. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Elisa Analyzer Market Growth Factors
The continual rise in lifestyle and chronic diseases globally is fuelling demand for Elisa Analyzers, especially to facilitate early disease diagnosis and management. For instance, a 2025 WHO report confirmed a 20% surge in autoimmune disease diagnoses, underlining the reliance on Elisa techniques. Technological advancements embedding automation and multiplexing capabilities are enhancing analyzer efficiency and throughput, which drives user adoption in pharmaceutical R&D sectors.
Expansion of government healthcare initiatives in emerging economies like India and China is increasing market penetration, with subsidized diagnostic infrastructures growing by over 30% annually since 2024. Additionally, the surge in personalized medicine and biomarker analysis propels market growth, with clinical trials extensively utilizing ELISA systems, contributing to a 25% increase in reagent and analyzer consumables revenue recorded in 2025.
Elisa Analyzer Market Development
In July 2024, Beckman Coulter Diagnostics introduced the DxC 500i Clinical Analyzer, an integrated clinical chemistry and immunoassay analyzer suited for satellite, independent, and core laboratories within healthcare networks. The DxC 500i features FlexMode operations that prioritize immunoassay or chemistry according to sample urgency, a dynamic sample handler that manages repeats and re-runs without operator intervention, and a compact footprint. It uses common reagents and consumables across Beckman Coulter’s scalable portfolio, enabling data commutability and simplified inventory management. The system supports a broad menu with more than 170 pre-programmed assays and is designed to deliver consistent patient results across networked laboratories.
In November 2023, DYNEX Technologies introduced the Agility® Integra system, a fully automated ELISA processor that is purpose-built for biotechnology and pharmaceutical laboratories and complies with U.S. FDA Regulation 21 CFR Part 11. The system supports high throughput by enabling multiple immunoassays to run simultaneously, incorporates advanced software functionalities including audit trails, chain-of-custody logging, data encryption, and secure networking, and features continuous sample loading and seamless LIMS integration. This launch positions DYNEX to serve the evolving needs of highly-regulated test environments where speed, data integrity, and automation are critical.
Key Players
Leading Companies of the Market
Thermo Fisher Scientific
Bio-Rad Laboratories
PerkinElmer, Inc.
Agilent Technologies
Siemens Healthineers
Merck KGaA
Abbott Laboratories
Promega Corporation
Molecular Devices LLC
QIAGEN N.V.
Becton Dickinson and Company
Several market players have adopted key growth strategies such as strategic acquisitions and product portfolio expansion to enhance their competitive positioning. For example, Thermo Fisher Scientific’s acquisition of PPD expanded its pharmaceutical testing capabilities, leading to a 15% boost in Elisa Analyzer sales in 2024. Likewise, Danaher’s emphasis on launching next-generation automated Elisa Analyzers contributed to a 12% revenue increase in the clinical diagnostics segment in 2025, improving its market share significantly.
Elisa Analyzer Market Future Outlook
The future of the market will be shaped by advancements in digital integration, automation, and connectivity. Cloud-based data analysis, AI-assisted assay validation, and modular systems that support multiplex testing will define the next generation of analyzers. The transition toward miniaturized and portable analyzers for point-of-care use is expected to open new avenues in decentralized diagnostics. Furthermore, partnerships between diagnostic manufacturers and software developers are likely to enhance workflow efficiency, while sustainability-focused innovations will prioritize reduced reagent waste and energy-efficient operations.
Elisa Analyzer Market Historical Analysis
The market has evolved significantly from manual microplate-based systems to highly automated analyzers designed for large-scale laboratory use. Historically, these systems were primarily used in research settings for enzyme-linked immunosorbent assays, but rising prevalence of infectious diseases and the growing need for precise diagnostic testing drove clinical adoption. Automation in the 2000s and 2010s improved throughput, minimized manual errors, and allowed integration with laboratory information systems. Continuous advancements in optical detection technologies, multi-assay compatibility, and reagent standardization have made ELISA analyzers indispensable in both hospital and reference laboratories.
Sources
Primary Research Interviews:
Clinical Laboratory Managers
Immunodiagnostic Specialists
Biomedical Engineers
Hospital Procurement Heads
Databases:
WHO In Vitro Diagnostics Data
ClinicalTrials.gov
PubMed Immunoassay Studies
Magazines:
Medical Device Network
Diagnostics World
Lab Manager
Clinical Lab Products
Journals:
Journal of Immunological Methods
Clinical Chemistry
Analytical Biochemistry
Journal of Laboratory Automation
Associations:
American Association for Clinical Chemistry (AACC)
International Federation of Clinical Chemistry (IFCC)
European Society for Laboratory Medicine (ESLM)
World Health Organization (WHO)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients