Needleless connectors are essential devices, which connect to the end of vascular catheters and enable catheter access for aspiration and infusion in patients. Needle-free intravenous catheter controls the access for vascular catheters. The use of needleless connectors reduces needle-stick injuries, which is major side effect of needle connectors and thus lessens the risk of blood borne infectious diseases. Needle-free connectors with extended dwell time can help to prevent intraluminal contamination, as well as device cross-contamination in patients.
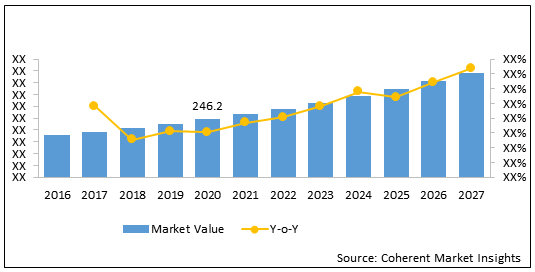
Europe needle-free IV connectors market is estimated to be valued at US$ 246.2 million in 2020 and is expected to exhibit a CAGR of 8.7% during the forecast period (2020-2027).
Figure 1. Europe Needle-free IV Connectors Market Value (US$ Mn), 2016-2027
To learn more about this report, Download Free Sample
Europe needle-free IV connectors market is expected to witness significant growth in the near future, owing to increasing needle-stick injuries caused by needle IV connectors. For instance, according to the European Agency for Safety and Health at Work (EU-OSHA) report 2018 estimates, around 1 million needle stick injuries (NSIs) are reported in Europe each year that are caused due to usage of needle connectors.
Moreover, key players in the market are focusing on launching of novel needle-free IV connectors, and launches and approval of such new needle-free IV connectors in the market are expected to support growth of the market during the forecast period. For instance, in September 2018, Amsino International, Inc., a U.S.-based medical device manufacturer, received the U.S. Food and Drug Administration (FDA) 510(k) approval for its new SURE-LOK needle-free connector, and it is also available in Europe.
Europe Needle-free IV Connectors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2020: | US$ 246.2 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 8.7% | 2027 Value Projection: | US$ 442.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Cardinal Health, Inc, Amsino International, Inc, ICU Medical, Inc, Vygon S.A, Baxter International Inc, Becton, Dickinson and Company, B. Braun Melsungen AG, NP Medical Inc, Poly Medicure Limited, CODAN Medizinische Geräte GmbH & Co KG, and Integrity Devices Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Europe Needle-free IV Connectors Market – Impact of Coronavirus (COVID-19)
Following the outbreak of COVID-19 in December 2019, the disease has spread to over 100 countries, across the globe and the World Health Organization has declared it as a public health emergency. According to the World Health Organization’s report, the manifestation of the coronavirus (COVID-19) has resulted in over 43.4 million infected individuals globally, as of October 27, 2020.
COVID-19 has affected the Europe needle-free IV connectors market in three main ways; by directly affecting production and demand, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as Italy, Germany, and the U.K. are facing challenges with regards to transportation of products and raw materials, which is expected to limit the market growth during the forecast period.
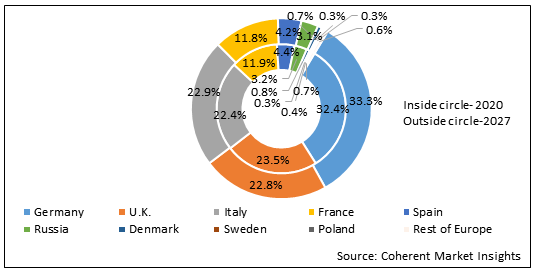
Figure 2. Europe Needle-free IV Connectors Market Share (%), by Country, 2020 & 2027
To learn more about this report, Download Free Sample
Among country, Germany is expected to witness significant growth in the Europe needle-free IV connectors market, owing to increasing risk of catheter-related bloodstream infections in ICUs in Germany, which is expected to drive demand for needle-free IV connectors in the market. For instance, according to the Antimicrobial Resist Infect Control and a study published by National Center for Biotechnology Information in 2018, the frequency of catheter-related bloodstream infections increased in ICUs in Germany between 2006 and 2015 and recorded 100 to 200 catheter-related bloodstream infection cases per 1,000 patients, each day.
Key Players
Major players operating in the Europe needle-free IV connectors market include Cardinal Health, Inc, Amsino International, Inc, ICU Medical, Inc, Vygon S.A, Baxter International Inc, Becton, Dickinson and Company, B. Braun Melsungen AG, NP Medical Inc, Poly Medicure Limited, CODAN Medizinische Geräte GmbH & Co KG, and Integrity Devices Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients