Orthopedic Extension Devices Market is estimated to be valued at USD 1,013.5 Mn in 2025 and is expected to reach USD 1,333.7 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.0% from 2025 to 2032.
Analysts’ Views on Global Orthopedic Extension Devices Market :
Increasing number of novel product approvals by the U.S. FDA are expected to boost the growth of the global orthopedic extension devices market over the forecast period. For instance, in February 2021, U.S. FDA approved the first patient-specific, 3D printed talus implant in the U.S. The agency issued approval to Additive Orthopaedics (A subsidiary of Paragon, Inc)- a U.S-based company specialized in developing cost-effective implant solutions, under humanitarian use for the treatment of avascular necrosis, a progressive condition that can lead to the death of bone tissue following a sudden injury that cuts off blood flow, such as a broken bone or dislocation.
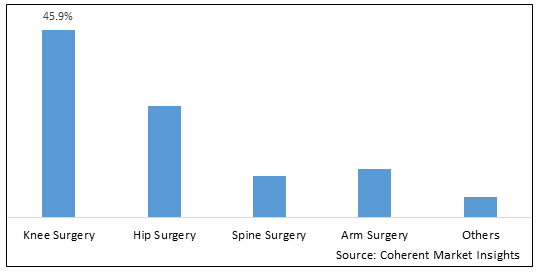
Figure 1. Global Orthopedic Extension Devices Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Source : Coherent Market Insights, 2025
Global Orthopedic Extension Devices Market – Driver
Increasing cases of injuries and trauma that require surgical procedures to boost the growth of the orthopedic extension devices market
The rising cases of trauma and injuries are expected to drive demand for surgical procedures and boost the growth of the orthopedic extension devices market. According to an article published in April 2020, in PubMed- a free search engine for biological databases, every year, over 310 million major orthopedic procedures are performed worldwide; approximately 40 to 50 million in the U.S. and 20 million in Europe. It is expected that 1-4% of these patients may die, up to 15% will experience severe postoperative morbidity, and 5-15% will be readmitted within 30 days. With an annual worldwide mortality rate of roughly 8 million patients, major surgery is comparable with the leading causes of death, which include cardiovascular disease and stroke, cancer, and accident.
Increasing product launches in orthopedic extension devices market
The increasing launch of products to invent newer orthopedic extension devices can drive the growth of the global orthopedic extension devices market over the forecast period. For instance, on May 16, 2023, Morristown Medical Centre of Atlantic Health System, a non-profit health care network in New Jersey, U.S., announced that were going to offer Persona IQ- the world’s first and only smart knee implant for Total Knee Replacement Surgery.
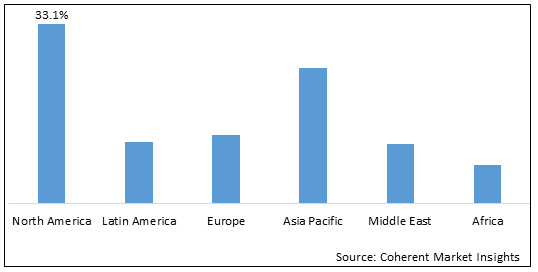
Figure 2. Global Orthopedic Extension Devices Market Value (US$ Million), by Region, 2025
To learn more about this report, Download Free Sample
Source : Coherent Market Insights, 2023
Global Orthopedic Extension Devices Market - Regional Analysis
North America held the dominant position in the global orthopedic extension devices market owing to the increasing number of surgical procedures in the region. According to the data published in May 2025 in Becker’s Orthopedic Review- a source offering news and analysis on business and legal issues relating to orthopedic and spine practices, nearly 1 million knee and hip arthroplasties were performed in 2021 the U.S.
Global Orthopedic Extension Devices Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
The COVID-19 pandemic had a negative impact on the global orthopedic extension devices market. According to a study published in May 2022, in PubMed, a free search engine for biological reports and databases, The COVID-19 pandemic reduced the amount of orthopaedic surgeries performed in 2020. During the first year of the pandemic, the number of orthopaedic procedures declined by 22.8%.
Global Orthopedic Extension Devices Market Segmentation:
The global orthopedic extension devices market report is segmented into product type, end user, and region.
By Product Type, The global orthopedic extension devices market is segmented into Hip Surgery, Knee Surgery, Spine Surgery, Arm Surgery and others. Out of which, the knee surgery segment is expected to hold a dominant position in the global orthopedic extension devices market during the forecast period and this is attributed to ease the pain of arthritis in older patients.
By End User, The global orthopedic extension devices market is segmented into Hospitals, Ambulatory Surgical Centers, and Specialty Clinics. Out of which, the Hospital segment is expected to dominate the market over the forecast period and this is attributed to the easy availability of sophisticated healthcare infrastructure in developed nations and the rising demand for high-quality healthcare services.
Based on Region, The global orthopedic extension devices market is segmented into North America, Europe, Asia Pacific, Latin America, Middle East & Africa out of which the North American segment is estimated to dominated the market over forecast period owing to the rising healtcare infrastructure and growing cases of major trauma in the region.
Among all the segmentations, the product type segment has the highest potential due to the increasing prevalence of osteoarthritis across the world over the forecast period. For instance, according to an article published in September 2021, in Cleaveland Clinic, a nonprofit, multispecialty academic medical center that integrates clinical and hospital care with research and education, approximately 46% of people develop osteroporosis of the knee during their lifetime after age 40.
Orthopedic Extension Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,013.5 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.0% | 2032 Value Projection: | USD 1,333.7 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Allen Medical Systems, Inc ( A subsidiary of Hill-Rom Holdings, Inc, a part of Baxter), AllianceImpex, Condor MedTec GmbH, DRE Medical (An Avante Health Solutions company), Implantech, IOT Innovative Orthopedic Technologies AG, Mediland Enterprise Corporation, MERIVAARA CORP, Mikai S.p.A, Mizuho OSI, Ningbo Techart Medical Equipment Co.,Ltd, OPT SURGISYSTEMS S.R.L, Schaerer Medical, SCHMITZ u. Söhne GmbH & Co. KG, SKYTRON, LLC, Smith & Nephew plc, St.Francis Medical Equipment Co., Ltd, TECHNOMED INDIA, TRUMPF |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Orthopedic Extension Devices Market Cross Sectional Analysis:
Increase in the cases of obesity in North America region is expected to drive growth of knee surgery segment. According to data published in September 2022, State of Obesity 2022- an annual report produced by Trust For America’s Health (TFAH) and the Robert Wood Johnson Foundation (RWJF), with support by a grant from RWJF, four in ten American adults have obesity. According to a report published on June 23, 2023, by The Blade, a U.S-based newspaper, an estimated 8,00,000 knee replacement procedures are done in the U.S. each year in which obesity plays a big role as obese people put all their weight through their knees. As a result, heavier patients increasingly need knee replacements at a younger age.
Global Orthopedic Extension Devices Market : Key Developments
On February 17, 2023, Curiteva, Inc., a spinal implant technology company based in the U.S, got U.S. FDA clearance for its Inspire 3D Porous PEEK Cervical Interbody System Technology. The implant technology exhibits good mechanical strength and helps to achieve a modulus of elasticity closely matching human cancellous bone.
On April 7, 2023, Ukraine’s civilian Emergency Medical Clinical Hospital collaborated with T&R Biofab, a U.S-based 3-D bioprinting specialist, to replicate special tissue microenvironments that would provided the ideal mileu for a skull implant that would be strong enough and that would enable the bone to grow back onto and into it.
On January 30, 2023, Additive Surgical, an Australia-based medical device manufacturer of 3D printed titanium, spine implant technology, launched a range of expandable interbody devices. The whole implant has a microporosity of 6-10 microns (surface texture) that creates a favorable environment for bony on-growth.
On January 27, 2023, Croom Medical, an outsourcing partner for the design and manufacture of orthopedic implants, introduced a 3D printed Sacroiliac Joint Fixation. The device provides biomechanical stability, support, and range of motions for patients suffering from SI joint dysfunction.
Global Orthopedic Extension Devices Market: Key Trends
On January 25, 2023, Trabtech, a Turkey-based technology company that provides training and consulting services in modeling and design, additive manufacturing as well as 3D anatomical modeling and design and customized solutions/services, invested US$ 1.3 million to fund the development of advanced 3D printed implant applications. With this funding, Trabtech will be able to complete the regulatory process for its new products, relocate its headquarters to Germany to enter the EU market and prepare for U.S. FDA approval, while commercializing its first mass-produced product, the TrabCup hip prosthesis.
In December 2021, Health Canada- the official drug regulatory authority of Canada, authorized the use of 3D printed mandibular implant to enable surgeons and medical professionals to use the Specific mandibular plate, along with surgical cutting and drilling guides, to treat patients. The implant reduces operation time and patient recovery time.
Global Orthopedic Extension Devices Market: Restraints
Product Recalls
Product recalls caused by defective goods or manufacturing faults reduce the rate of acceptance of these devices by surgeons and patients, lowering manufacturers' revenues. Challenges with size fittings, composition or other negative impacts of the devices are can hamper the growth of the global orthopedic extension devices market. For instance, in July 2020, Zimmer Biomet, a publicly traded medical device company, recalled an unspecified number of polyethylene orthopedic implants in Europe due to the possible presence of elevated endotoxin levels. Strict compliance with good manufacturing practices and regulatiory guidelines can help reduce the frequency of manufactural defects in products.
High cost of medical implants
The high costs of orthopedic implants and devices is estimated to slow down the the growth of the global orthopedic extension devices market. For instance, according to an article published by The Asian Journal Of Pharmaceutical Sciences, the official journal of Asian Federation for Pharmaceutical Sciences, on May 15, 2023, beyond the warranty period, even a small repair in cochlear implants ranges between US$ 600 to US$ 1000. According to the American Association Of Hip And Knee Surgeons, the price for most primary hip and replacement parts generally range from US$ $3,000 - $10,000. Medical insurances, government initiatives and surgeries at tertiary medical centres can serve as an effective counterbalance for this restraint.
Global Orthopedic Extension Devices Market - Key Players
Major players operating in the global orthopedic extension devices market Allen Medical Systems, Inc ( A subsidiary of Hill-Rom Holdings, Inc, a part of Baxter), AllianceImpex, Condor MedTec GmbH, DRE Medical (An Avante Health Solutions company), Implantech, IOT Innovative Orthopedic Technologies AG, Mediland Enterprise Corporation, Merivaara Corp, Mikai S.p.A, Mizuho OSI, Ningbo Techart Medical Equipment Co.,Ltd, OPT SURGISYSTEMS S.R.L, Schaerer Medical, SCHMITZ u. Söhne GmbH & Co. KG, SKYTRON, LLC, Smith & Nephew plc, St.Francis Medical Equipment Co., Ltd, TECHNOMED INDIA, TRUMPF.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients